Values for the molar mass of hydrogen, oxygen, and water molecules are
given in the table below. What mass of water is formed when 2 moles of
hydrogen react with 1 mole of oxygen to form water?
Molecule
Molar mass (g/mol)
H2
2.02
02
32.00
H20
18.01
A.9.00 g
B. 36.02 g
C. 2.00 g
D. 18.01 g
Answers
Answer:
36.02g bbbbbbbbbbb hbbnjkkkj
Answer:
36.02g
Explanation:
Hope this helps!
Related Questions
Which group has the highest ionization energies? Explain why.
Answers
Answer:
nobel elements (gr. 18) because they are fully stable due to octet complete--------------------------------------------------
How many grams are equal to 413 kg in scientific notation
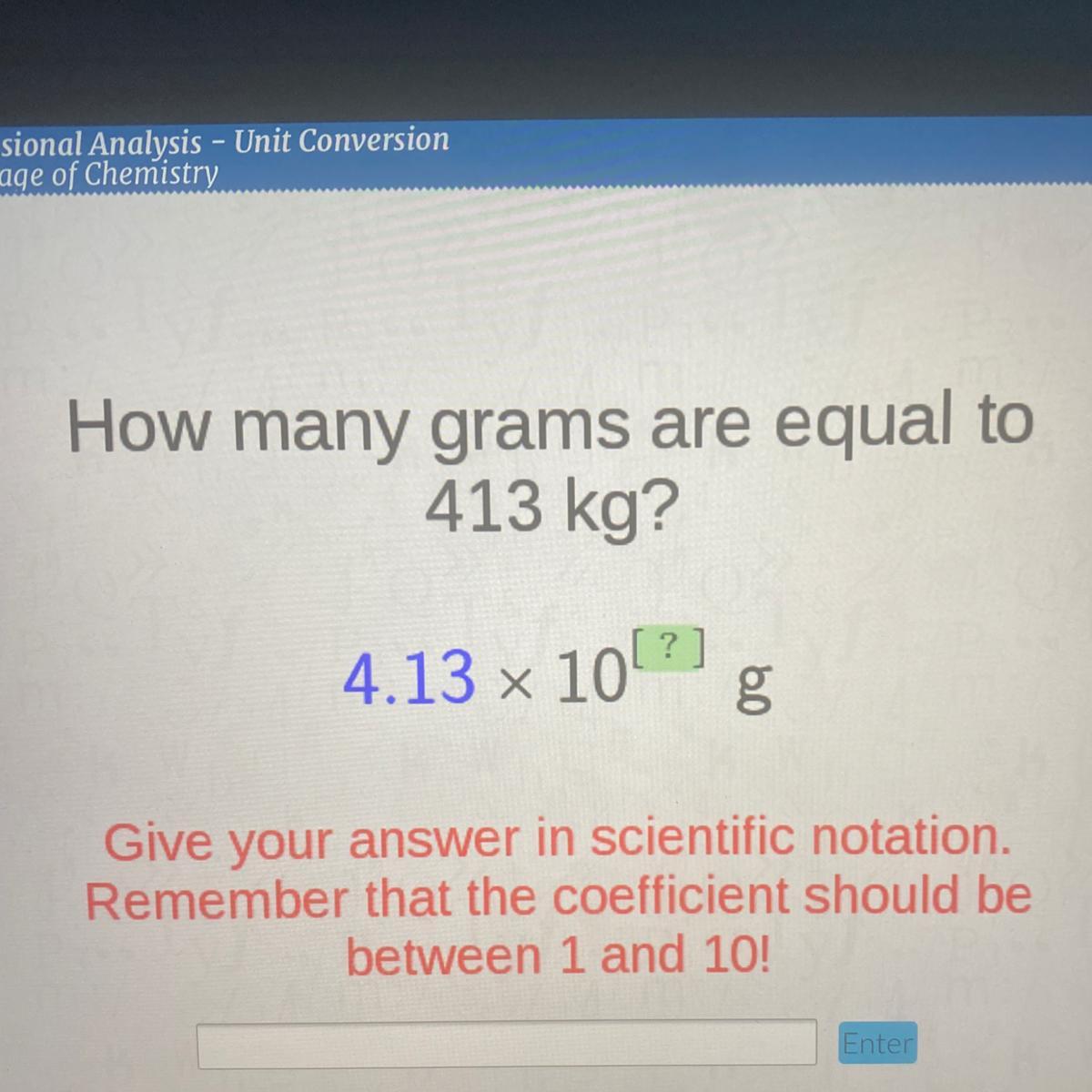
Answers
Answer:
\(4.13\) x \(10^{2}\) = 413 kg
Explanation:
4.13 x 10 = 41.3 then we multiply 41.3 by 10 agian = 41.3 x 10 = 413
so, we multiplied ten two times that = \(10^{2}\)
= 4.13 x \(10^{2}\)= 413 kg
Given that 1 kilogram = 1000grams, the value of 413 kilograms in grams, using scientific notation is 4.13 × 10⁵g.
How many grams are equal to 413 kg in scientific notation?Note that;
1 kilogram = 1000grams
Given that;
Mass in kilograms = 413kgMass in grams = ?Since, 1 kilogram = 1000grams
413 kilograms = ( 413 × 1000 )grams
413 kilograms = ( 413000 )grams
413 kilograms = 4.13 × 10⁵ g
Given that 1 kilogram = 1000grams, the value of 413 kilograms in grams, using scientific notation is 4.13 × 10⁵g.
Learn more about conversion of mass here: https://brainly.com/question/14147088
#SPJ9
the ocean pressure at the depth of the titanic wreck is 400 atm . calculate the ocean pressure in kpa. round answer to significant digits.
Answers
\(4.05 * 10 ^{4}\)
To find the pressure in kPa we use the conversion
1 atm = 101.3 kPa
If 1 atm = 101.3 kPa
400 atm = 400 × 101.3 = 40520 kPa
Expressing it in standard form we have the final answer as
4.05 × 10⁴ kPa
Pressure (symbol: p or P) is the force applied perpendicular to the surface of an object per unit area over which that force is distributed. Pressure may also be expressed in terms of standard atmospheric pressure; the atmosphere (atm) is equal to this pressure, and the torr is defined as 1⁄760 of this. Manometric units such as the centimeter of water, millimetre of mercury, and inch of mercury are used to express pressures in terms of the height of column of a particular fluid in a manometer.
P = F / A
To know more about standard atmospheric pressure click here:
https://brainly.com/question/14362967
#SPJ4
Explain why, in a balanced chemical equation C + O2 CO2, we know that 1 gram of C will not react exactly with 1 gram of O2.
Answers
Answer:
See the answer below
Explanation:
From the equation, 1 mole of C requires 1 mole of O2 for a balanced reaction.
1 g of C will not exactly react with 1 g of O2 because the 1 g of each of the reactants contain different amounts of the substance.
The amount (in moles) of a substance is the mass of that substance divided by the molar mass.
Hence, 1 g of C will contain 1/12 moles of the substance while 1 g of O2 will contain 1/32 moles of the substance.
dan collected approximately 10 milliliters of waste acetone during an experiment in chemistry 101. what actions should dan take to properly dispose of the waste acetone?:
Answers
Dan should be responsible and know that hazardous waste needs proper disposal. He should be aware of the proper disposal methods of waste acetone to keep the environment and humans safe.
Dan collected approximately 10 milliliters of waste acetone during an experiment in chemistry 101.
To properly dispose of the waste acetone, Dan should follow these actions:Label:
Label the waste container properly and correctly with the chemical name acetone and a hazardous waste label.
This would help others know what the container contains, and it will prevent confusion in case another substance is also in the vicinity.
Tighten the container: Ensure that the cap of the container is tightly closed.
This will prevent the acetone from evaporating, spilling or leaking out from the container.
Proper Storage: Dan should store the waste acetone in a cool, dry and well-ventilated area, away from direct sunlight. Flammable materials should not be near the container as acetone is flammable.
He should not store it in metal containers, as acetone can corrode metal containers.
Contact Authorities: Dan should contact the authorities in charge of hazardous waste disposal in his area to know the proper way to dispose of waste acetone. In some places, they have specialized collection sites for hazardous waste.
He should never pour waste acetone down the drain, on the ground, or in the trash.
Dan should be responsible and know that hazardous waste needs proper disposal. He should be aware of the proper disposal methods of waste acetone to keep the environment and humans safe.
To know more about hazardous waste, visit:
https://brainly.com/question/24043586
#SPJ11
Assume we have 759 liters of N, at ST. What is the mass of the nitrogen gas? Give answers to the nearest whole number.
Answers
So, at STP, there are around 891 grammes of nitrogen gas in every 759 litres.
Which mass is greater, 14 or 15?The two stable isotopes of naturally occurring nitrogen (7N) are nitrogen-14 and nitrogen-15, with nitrogen-14 constituting 99.6% of all naturally existing nitrogen. Along with one nuclear isomer, 11mN, fourteen radioisotopes with atomic masses ranging from 10 to 25 are also known.
Assuming "ST" refers to standard temperature and pressure (0°C and 1 atm), we can use the ideal gas law to calculate the mass of nitrogen gas:
PV = nRT
where n is the number of moles, P is the pressure, V is the volume, and T is the temperature, and R is the gas constant.
The temperature and pressure are 273.15 K and 1 atm, respectively, at STP.
To solve for n, the number of moles, we can rearrange the ideal gas law equation as follows:
n = PV / RT = (1 atm) * (759 L) / (0.0821 L·atm/(mol·K) * 273.15 K) = 31.8 mol
Now we can calculate the mass of nitrogen gas:
mass = n * molar mass = 31.8 mol * 28.01 g/mol ≈ 891 g.
To know more about nitrogen gas visit:-
https://brainly.com/question/13907528
#SPJ1
Consider the equilibrium system of cobalt complexes. Co(H20) 2+ (aq) + 4C1- (aq) = CoCl2- (aq) + 6H2O(1) The Co(H20)62+ (aq) complex is pink and the CoC12- (aq) complex is light blue. Determine what each color observation means about changes made to the system at equilibrium. The solution changes from pink to light blue. Choose... The solution changes from light blue to pink. Choose... The solution stays light blue after adding a chemical. Choose..
Answers
The color change of the equilibrium system of cobalt complexes can provide valuable information about changes made to the system at equilibrium. In this case, the Co(H₂0)₆²⁺ (aq) complex is pink and the CoCl₂⁻ (aq) complex is light blue.
If the solution changes from pink to light blue, it means that the concentration of CoCl₂⁻ (aq) complex has increased and the concentration of Co(H₂0)₆²⁺ (aq) complex has decreased. This could be due to the addition of more chloride ions or the removal of water molecules from the system. As a result, the equilibrium shifts towards the side of the equation with fewer chloride ions and more water molecules.
On the other hand, if the solution changes from light blue to pink, it means that the concentration of Co(H₂0)₆²⁺ (aq) complex has increased and the concentration of CoCl₂⁻ (aq) complex has decreased. This could be due to the addition of more water molecules or the removal of chloride ions from the system. As a result, the equilibrium shifts towards the side of the equation with fewer water molecules and more chloride ions.
If the solution stays light blue after adding a chemical, it means that the added chemical has no effect on the equilibrium system. This could be because the added chemical does not react with any of the species in the equilibrium system or because its effect is negligible compared to the existing concentrations of the species.
To know more about equilibrium, refer to the link below:
https://brainly.com/question/15398944#
#SPJ11
which of the following keeps satellite in orbit
Answers
Answer:
Gravity
Explanation:
Without gravity it would just float in space.
Please mark brainliest, i need two more to achieve expert
A 0.80 L sample of gas has a temperature of 27°C and a pressure of 0.925 atm. How many moles of gas are present?
Answers
Answer:
n= 0.03 moles
Explanation:
Using the ideal gas law:
PV=nRT
nRT=PV
n= PV/RT
n: moles
P: pressure in atm
V= volume in L
R= Avogadro's constant = 0.0821
T= Temperature in K => ºC+273.15
n= (0.925 atm)(0.80 L) / (0.0821)(300.15 K)
n= 0.03 moles
how many moles of al(cn)3 are in 231 g of the compound?
Answers
Answer:
About 2.20 moles.
Explanation:
We want to determine the amount of moles of aluminum cyanide in 231 grams of the substance.
To do so, we can convert from moles to grams using its molar mass.
Find the molar mass of Al(CN)₃:
\(\displaystyle \begin{aligned} \mathcal{M}_{\text{Al(CN)$_3$} } & = \left( \underbrace{26.98}_{\text{Al}} + 3(\underbrace{12.01}_{\text{C}}) + 3(\underbrace{14.01}_{\text{N}})\right) \text{ g/mol} \\ \\ & =105.04\text{ g/mol} \end{aligned}\)
With the initial value, multiply:
\(\displaystyle 231 \text{ g Al(CN)$_3$} \cdot \frac{1 \text{ mol Al(CN)$_3$}}{105.04\text{ g Al(CN)$_3$}} = 2.20\text{ mol Al(CN)$_3$}\)
In conclusion, there are about 2.20 moles of aluminum cyanide in 231 grams of the substance.
Read the following reactions.
Reaction 1: NaCl(s) → Na+(aq) + Cl−
Reaction 2: CaCO3(s) → CaO(s) + CO2(g)
Which reaction leads to an increase in entropy?
a
Only Reaction 1
b
Only Reaction 2
c
Both Reaction 1 and 2
d
Neither Reaction 1 nor 2
Answers
Answer: C
Explanation:
Reaction 1 starts off with one mole of reactant and produces 2 moles of product. The increase in the number of moles, along with the fact that aqueous compounds are more disordered than solid compounds, increases the disorder and thus entropy.
Reaction 2 starts off with 1 mole of solid and produces 1 mole of solid and one mole of gas. The increase in the number of moles, along with the fact that gases are more free and disordered than solids, increases the disorder and thus entropy.
If a book has a weight of 23.2 N on Earth, what is its mass?
Answers
Answer: Mass is 2,37 kg
Explanation: Weight G = mg, and g = 9.81 m/s² on Earth.
m = W/g = 23.2 N / 9.81 m/s²
ito ang kontinenteng nagtataglay ng pinakamaraming bansa sa lahat ng kontinente
Answers
Answer:
Africa
Explanation:
Ang Africa ay ang kontinente na mayroong pinakamataas na bilang ng mga bansa sa lahat ng mga kontinente.
Kasalukuyan, ang bilang ng mga bansa sa kontinente ay 54, ito ay 53 bago ang South Sudan ang pinakabagong sumali.
Ang bilang na ito ay pinaniniwalaang tataas sa mga darating na taon dahil sa kaguluhan ng iba't ibang mga segment sa ilang mga bansa para sa kalayaan
Express 36,000,000 in scientific notation
Answers
Answer:
\(3.6 * 10^{7}\)
Explanation:
Hope this helps!
What is the notation for the enthalpy of solution?
O -Hsol
O AH sol
Ο ΔΕ
O +Hsol
Answers
The notation for the enthalpy of the solution is ∆Hsol. The correct answer is option ∆Hsol.
The enthalpy of solution is a measure of the amount of heat absorbed or released when a solute is dissolved in a solvent to form a solution. If the value of ∆Hsol is positive, it means that heat is absorbed during the process of dissolving the solute, while a negative value of ∆Hsol indicates that heat is released during the same process. This value is often used to predict whether a given solute will dissolve in a given solvent, as well as the relative amounts of solute and solvent that will be required to form a solution. The enthalpy of solution can be calculated experimentally by measuring the temperature change that occurs when a known amount of solute is dissolved in a known amount of solvent. Alternatively, it can be calculated theoretically using thermodynamic data for the solute and solvent.For more questions on enthalpy
https://brainly.com/question/14047927
#SPJ8
How many moles are in 48.1 grams of FeF3?

Answers
Answer:
I am not sure which number would be considered correct.
unrounded: 5,427.5559
rounded to the nearest thousandth: 5,427.556
rounded to the nearest hundredth: 5,427.56
rounded to the nearest tenth: 5,427.6
rounded to the nearest whole number: 5,428
Explanation:
112.839 * 48.1 = 5,427.5559
How do animal behaviors, such as hibernation or migration, enhance their survival?
Answers
Hibernation and migration are adaptations that animals use to sustain life during the winter months when demand outstrips supply. Hibernation occurs when animals rest or sleep for the entire winter. The movement of animals from one location to another is referred to as migration.
What is hibernation?Hibernation is a tactic by which animals conserve energy to survive in adverse weather conditions or a lack of food.
It is characterized by physiological changes such as a decrease in body temperature and a slowing of metabolism.
The movement of animals from one location to another is referred to as migration.
Hibernation and migration are adaptations that animals use to sustain life the winter months when demand outstrips supply.
Thus, animal behaviors, such as hibernation or migration, enhance organisms' chances of survival.
For more details regarding Hibernation, visit:
https://brainly.com/question/1884191
#SPJ2
Given the unbalanced equation: Al2(SO4)3 + Ca(OH)2 + Al(OH)3 + CaSO4 What is the coefficient in front of the CaSO4 when the equation is completely balanced with the smallest whole-number coefficients? A. 1 B. 2 C. 3 D. 4
Answers
The balanced equation with the smallest whole-number coefficients is Al2(SO4)3 + 3Ca(OH)2 → 2Al(OH)3 + 3CaSO4. Therefore, the coefficient in front of the CaSO4 when the equation is completely balanced with the smallest whole-number coefficients is 3 (option C).
To determine the coefficient in front of the CaSO4 when the given unbalanced equation Al2(SO4)3 + Ca(OH)2 → Al(OH)3 + CaSO4 is completely balanced with the smallest whole-number coefficients, follow these steps:
Balance the aluminum (Al) atoms: Place a coefficient of 2 in front of Al(OH)3. Now the equation is:Al2(SO4)3 + Ca(OH)2 → 2Al(OH)3 + CaSO4Balance the sulfur (S) atoms: The equation is already balanced for sulfur atoms.Balance the oxygen (O) atoms: The equation is balanced for oxygen atoms as well.Balance the calcium (Ca) atoms: Place a coefficient of 3 in front of Ca(OH)2 and a coefficient of 3 in front of CaSO4. Now the equation is:
Al2(SO4)3 + 3Ca(OH)2 → 2Al(OH)3 + 3CaSO4
See more about equation in:
https://brainly.com/question/2972832
#SPJ11
Why is it important to choose a relevant scale for analyzing data sets in science?
(Will mark you brainliest)
Answers
Answer:
It is critical to understand how the numbers allocated to entities, objects, and events are to be interpreted. Data collection, analysis, and presentation all need the use of a measurement scale. We should utilize the correct diagram for the data collection, which is highly important for expressing summaries and conclusions to the audience quickly and simply.
Tyler measured the force of his grip. Which is the most likely reading?
A.
190kg
B.
190N
C.
1 lb
D.
20,000 N
E.
5 seconds
F.
18mg
Answers
Answer:
190n
Explanation:
190 190 and because it is easy to understand if you didn't understand my answer ask any man
which is a metal that becomes isoelectronic with krypton ?
Answers
A metal which becomes isoelectronic with Krypton when it forms its most common ion is Strontium (Sr2+). The correct option is E.
What is isoelectronic?Isoelectronic species are atoms, elements, ions, or molecules that have an equal number of valence electrons and the same atom connectivity. For example, Carbon Monoxide (CO) and Dinitrogen (N2) are isoelectronic, because both have ten valence electrons.
In this case, from the periodic table we know that Krypton (Kr) has an atomic number equal to 36, which means that a neutral Krypton atom has 36 electrons surrounding its nucleus. Consequently, any species which is isoelectronic with Krypton also have 36 electrons surrounding its nucleus.
For Calcium (Ca), it has 20 electrons. It means Ca2+ will have 2 electrons less, or 18 electrons. So, it is not isoelectronic with Krypton.
For Potassium (K), it has 19 electrons. It means K+ will have 18 electrons. So, it is not isoelectronic with Krypton.
For Iodine (I), it has 53 electrons. It means I- will have 54 electrons. So, it is not isoelectronic with Krypton.
For Magnesium (Mg), it has 12 electrons. It means Mg2+ will have 10 electrons. So, it is not isoelectronic with Krypton.
For Strontium (Sr), it has 38 electrons. It means Sr2+ will have 36 electrons. So, it is isoelectronic with a neutral Krypton atom.
Hence, the one which becomes isoelectronic with Krypton is Strontium (Sr2+).
Learn more about isoelectronic at: https://brainly.com/question/6807313
#SPJ4
Although part of your question is missing, you might be referring to this full question: Which is a metal that becomes isoelectronic with Krypton when it forms its most common ion?
a. Ca2+
b. K+
c. I−
d. Mg2+
e. Sr2+
Balance the following reactions:'
Mg(OH)2 + HCl ➡️ H2O + MgCl2
Ca(ClO3)2 ➡️ CaCl2 + O2
Ca(NO3)2 + ➡️ KBr CaBr2 + KNO3
Al + HCl ➡️ AlCl3 + H2
C2H6 + O2 ➡️ CO2 + H2O
Answers
\(2 HCl + Mg(OH)_2\) → \(2 H_2O + MgCl_2\)
\(3 Ca(ClO)_2\) → \(2 CaCl_2\) + \(Ca(ClO_3)_2\)
\(2 KBr + Ca(NO_3)_2\) →\(2 KNO_3 + CaBr_2\)
\(2Al +6HCl\) → \(2AlCl_3 +3H_2\)
\(2C_2H_6+7O_2\) → \(4CO_2+6H_2O.\)
What is a balanced chemical equation?A balanced chemical reaction is an equation that has equal numbers of each type of atom on both sides of the arrow.
Balanced chemical equation:
\(2 HCl + Mg(OH)_2\) → \(2 H_2O + MgCl_2\)
\(3 Ca(ClO)_2\) → \(2 CaCl_2\) + \(Ca(ClO_3)_2\)
\(2 KBr + Ca(NO_3)_2\) →\(2 KNO_3 + CaBr_2\)
\(2Al +6HCl\) → \(2AlCl_3 +3H_2\)
\(2C_2H_6+7O_2\) → \(4CO_2+6H_2O.\)
Learn about the balanced chemical equation here:
https://brainly.com/question/24245953
#SPJ1
Use linear algebra to balance the chemical equation: C7H₁6 +0₂ → CO₂ + H₂O. 20. Let V be the set of all vectors in ³ whose components sum to zero (e.g. (-5, 2, 3) is in the set V but (0, 0, 1) is not). Is V a subspace of R³2 Give compelling evidence either way. 15. (Determine the quadratic interpolant to the given data set using linear algebraic techniques. (The quadratic interpolant is a quadratic equation that best approximates the data set). {(6.667, 46.307), (4.567, 16.582), (3.333, 4.857)}
Answers
The balanced chemical equation is:
0.5C7H16 + O2 → 0.5CO2 + H2O
For balancing the chemical equation C7H16 + O2 → CO2 + H2O, we can use linear algebraic techniques. We need to determine the coefficients that balance the number of atoms on both sides of the equation.
Let's denote the coefficients for C7H16, O2, CO2, and H2O as a, b, c, and d, respectively.
The balanced chemical equation can be written as:
aC7H16 + bO2 → cCO2 + dH2O
To balance the carbon (C) atoms, we have:
7a = c (Equation 1)
To balance the hydrogen (H) atoms, we have:
16a = 2d (Equation 2)
To balance the oxygen (O) atoms, we have:
2b = 2c + d (Equation 3)
We have three equations (Equations 1, 2, and 3) and four unknowns (a, b, c, d). To solve this system of equations, we can write it in matrix form and find the solution using linear algebraic techniques.
The augmented matrix for the system of equations is:
[ 7 0 -1 0 | 0 ]
[ 0 0 0 -2 | 0 ]
[ 0 -2 2 -1 | 0 ]
By performing row operations to row-reduce the augmented matrix, we can obtain the solution:
[ 1 0 -0.5 0 ]
[ 0 1 -1 -0.5 ]
[ 0 0 0 0 ]
The solution to the system of equations is:
a = 0.5
b = 1
c = 0.5
d = 1
Putting the values of a,b,c, and d we get the balanced chemical equation as:
0.5C7H16 + O2 → 0.5CO2 + H2O
To learn more about balancing, visit:
https://brainly.com/question/31242898
#SPJ11
How many moles of sodium carbonate in 18.06x10 to the power 22
Answers
Answer:
\( moles = \frac{ number \: of \: particles}{6.02 \times {10}^{23} } \)
=1.806×10^22/6.02×10^23
=0.03 moles
hope this helps :)
Explanation none
Combining 0.342 mol Fe2O3 with excess carbon produced 12.5 g Fe
Fe2O3 + 3 C -> 2Fe + 3 CO.
What is the actual yield of iron in moles? actual yield: mol What is the theoretical yield of iron in moles? theoretical yield: mol What is the percent yield? percent yield:
Answers
The actual yield of iron is 0.223 mol Fe.
The theoretical yield of iron is 0.684 mol Fe.
The percent yield is 32.6%.
The balanced chemical equation is Fe₂O₃ + 3C -> 2Fe + 3CO.
First, we need to calculate the theoretical yield of Fe. We know that 0.342 mol Fe₂O₃ is used, and the molar ratio between Fe₂O₃ and Fe is 1:2 (from the balanced equation). Therefore, the theoretical yield of Fe is:
0.342 mol Fe₂O₃ x (2 mol Fe / 1 mol Fe₂O₃) = 0.684 mol Fe
Next, we need to calculate the actual yield of Fe. We know that 12.5 g Fe was produced, and we can convert that to moles using the molar mass of Fe:
12.5 g Fe x (1 mol Fe / 55.845 g Fe) = 0.223 mol Fe
Finally, we can calculate the percent yield:
percent yield = (actual yield / theoretical yield) x 100
percent yield = (0.223 mol Fe / 0.684 mol Fe) x 100
percent yield = 32.6%
Therefore, the actual yield of Fe in moles is 0.223 mol, the theoretical yield of Fe in moles is 0.684 mol, and the percent yield is 32.6%.
Learn more about percent yield here: https://brainly.com/question/25996347
#SPJ11
how many elements are there in magnesium chloride, mgcl2?
Answers
Magnesium chloride, MgCl₂, is composed of 3 elements: magnesium (Mg), chlorine (Cl), and two atoms of chlorine (Cl).
An element is a basic substance that cannot be broken down into simpler substances by chemical means. Each element is defined by its atomic number, which is equal to the number of protons in the nucleus of its atom. There are 118 known elements, ranging from the lightest, hydrogen, to the heaviest, oganesson. Elements can combine to form compounds, which have unique properties and characteristics. Some elements, such as carbon and oxygen, form the building blocks of life, while others, such as gold and silver, have been valued for their beauty and rarity. The properties of elements are determined by the arrangement of their electrons, and these properties can be used to predict the behavior of elements in chemical reactions.
Learn more about elements:
brainly.com/question/13025901
#SPJ4
what is the chemical reaction when a gas, liquid, and solid mix? EASY POINTS ANSWER TO A BRAINLIEST
Answers
PLEASE HELP!!!
Which of the analogies best describes a Bohr model of an atom?
A. A Bohr model is like a bowling ball because they are both solid spheres.
B.A Bohr model is like a model of the solar system because they both show orbits around a massive center
C. A Bohr model is like a string of beads because they both contain small parts that are lined up in a row.
D. A Bohr model is like a jigsaw puzzle because they are both made up of small parts that are all joined together.
Answers
Answer:
B is the Bohr model of atom
How do we solve this? I got B but answer key says A

Answers
The concentration of NH3 at equillibirium is 0.00010M. Option A.
Ammonium nitrate is formed when nitric acid reacts with ammonia. It is a white crystalline solid consisting of ammonium ions and nitrate ions. Soluble in water, but does not form hydrates. Ammonia is directly neutralized with sulfuric acid to produce ammonium sulfate.
The neutralization evaporator and crystallizer are connected so that the heat released during neutralization is used to evaporate the water in the ammonium sulfate slurry. These units operate under partial vacuum. Nitric acid pH neutralization is common and any inorganic base such as sodium hydroxide or lime can be used. Ammonia gas reacts with hydrogen chloride gas to form ammonium chloride.
Learn more about Nitrous acid here:-https://brainly.com/question/25752475
#SPJ9
a graduated cylinder with a mass of 105.56 g has 45.4 ml of a certain liquid added to it. the mass of the cylinder and the liquid is 136.15 g. what is the density of this liquid?
Answers
The density of the liquid is 0.794 g/mL.
To calculate the density of the liquid, we can use the formula:
Density = Mass / Volume
Given information:
Mass of the graduated cylinder = 105.56 g
Volume of the liquid = 45.4 mL
Total mass of cylinder and liquid = 136.15 g
To find the mass of the liquid, we subtract the mass of the cylinder from the total mass:
Mass of the liquid = Total mass - Mass of the cylinder
Mass of the liquid = 136.15 g - 105.56 g
Mass of the liquid = 30.59 g
Now we can calculate the density:
Density = Mass of the liquid / Volume of the liquid
Density = 30.59 g / 45.4 mL
Converting mL to cm³ (since 1 mL = 1 cm³):
Density = 30.59 g / 45.4 cm³
Density = 0.674 g/cm³
Rounding to three decimal places, the density of the liquid is approximately 0.794 g/mL.
Learn more about density: https://brainly.com/question/1354972
#SPJ11