assuming all of these molecules have the same number of carbon atoms, which functional group would have the highest boiling point?
Answers
The correct answer is
Aldehyde would have the highest boiling point
Aldehydes and ketone both include a carbonyl group. Aldehydes are thought to be the most important functional group. They go by the labels formyl or methanoyl group. Aldehydes get their name from the dehydration of alcohols. Aldehydes have a carbonyl group attached to at least one hydrogen atom. In ketones, the carbonyl group is joined to two carbon atoms.
Examples of organic compounds with the carbonyl functional group, or C=O, include aldehydes and ketones. The carbon atom of this group has two empty bonds that may be filled with hydrogen, an alkyl group, or an aryl group. If at least one of these substituents is hydrogen, the compound is an aldehyde. If none of these contains hydrogen, the material is a ketone.
To learn more about Aldehyde click the link below
brainly.com/question/30459994
#SPJ4
Related Questions
N. 39.8 g HzF to L. unit convert
Answers
Answer: 39.9 g HzF is equivalent to 0.0398 L.
Explanation:
According to the standard conversion factor,
1 gram = 0.001 L
Therefore, 39.8 g will be converted into liter as follows.
\(1 g = 0.001 L\\39.8 g = 39.8 g \times \frac{0.001 L}{1 g}\\= 0.0398 L\)
Thus, we can conclude that 39.9 g HzF is equivalent to 0.0398 L.
how is an object's speed determined?
Answers
Answer:
Divide the distance the object traveled by the time it took to get there.
Explanation:
To calculate the speed on an object, start by determining how far the object has traveled. Next, figure out the amount of time that the object took to cover that distance. Finally, divide the distance the object traveled by the time it took to get there. Don't forget to label the speed with the correct units of measurement.

Which of the following could affect infiltration of water into the surface of the soil? Explain in detail.
I. Concrete sidewalks
II. Parks and gardens
I WILL MARK AS BRAINLIST
Answers
Answer:
I. Concrete sidewalks
Explanation:
One of the factor that affects the infiltration of water into the surface of the soil is the use of concrete sidewalks.
A concrete sidewalk prevents water from infiltrating down into the soil profile. Infiltration deals with the movement of water into the soil or ground. It is the major source of ground water recharge. Also, infiltration makes water available for plants.Concrete is impermeable and non - porous media
When concrete pavements are used, water will not be able to move into the ground. They run on the surface and collects into nearby streams and water bodies.
What happen to rainwater after it falls? Where does it goes?
Answers
Answer:
of the Drain! What happens to rain after it falls? Rainwater, or snow melt, either soaks into the ground to become groundwater, evaporates, or flows over the surface of the land. The water that flows over the ground is called stormwater or runoff.
A lump of zinc is tossed into a beaker of 500L of 14M hydrochloric acid. this reaction produces Hydrogen Gas and zinc (II) chloride. If the hydrogen gas is combusted and produces 645L of water vapor at 400 kelvin and 1.75 atm, what is the mass of the zinc?
Answers
If the hydrogen gas is combusted and produces 645L of water vapor at 400 kelvin and 1.75 atm, 2796.96 g mass of the zinc is produced .
Using the ideal gas law equation:
PV = nRT
n = (PV) / (RT)
= (1.75 atm * 645 L) / (0.0821 atm·L/(mol·K) * 400 K)
= 42.71 moles
the balanced equation for the reaction between zinc and hydrochloric acid:
Zn + 2HCl -> \(ZnCl_{2}\) + \(H_{2}\)
1 mole of zinc produces 1 mole of hydrogen gas. Therefore, the moles of zinc are also 42.71.
The molar mass of zinc is 65.38 g/mol.
Mass of zinc = moles of zinc * molar mass of zinc
= 42.71 moles * 65.38 g/mol
= 2796.96 g
Therefore, the mass of the zinc is 2796.96 grams.
learn more about hydrogen gas :
https://brainly.com/question/30829657
A spring with a spring constant of 3N/m is stretched until extended by 1.4m. How much elastic potential energy is stored by the spring?
Give your answer to 2 decimal places
Answers
The energy that is stored in the spring is 2.94 J.
What is the elastic potential energy?We have to note that the elastic potential energy of the spring is the energy that has been stored in the spring and it is released to be able to do work once we pull the spring.
In this case we already know that;
W = 1/2Ke^2
W = work done
K = force constant
e = extension
Thus;
W = 0.5 * 3 * 1.4^2
W = 2.94 J
We can see that the stored energy for the spring in question is 2.94 J of energy stored.
Learn more about spring constant:https://brainly.com/question/14159361
#SPJ1
Does anybody understand this I’m stuck

Answers
Answer:
it's the first one
Explanation:
can you help me too
18.A helium balloon has a volume of 3.0 m^3at lift off where the air pressure is 1 atm and the temperature is 20°C. When airborne, the temperature decreases to −60°C and the volume expands to 120 m^3. What it the pressure at this alriftide?
Answers
The pressure when the helium balloon is airborne at a volume of 120 m³ and a temperature of -60°C is approximately 0.726 atm.
To solve this problem, we can use the ideal gas law, which states that:
PV = nRT
P is the pressure
V is the volume
n is the number of moles of gas
R is the ideal gas constant (8.314 J/(mol·K))
T is the temperature in Kelvin
First, let's convert the initial and final temperatures from Celsius to Kelvin:
Initial temperature (T1) = 20°C + 273.15 = 293.15 K
Final temperature (T2) = -60°C + 273.15 = 213.15 K
Next, we can set up two equations using the ideal gas law for the initial and final states:
P1 * V1 = n * R * T1
P2 * V2 = n * R * T2
Since the number of moles (n) and the gas constant (R) are constant, we can write:
P1 * V1 / T1 = P2 * V2 / T2
Now we can plug in the given values:
P1 * 3.0 m³ / 293.15 K = P2 * 120 m³ / 213.15 K
Simplifying the equation:
P1 / 293.15 = P2 / 213.15
Now we can solve for P2:
P2 = P1 * 213.15 / 293.15
Finally, we can substitute the initial pressure (P1) with the given value of 1 atm:
P2 = (1 atm) * 213.15 / 293.15
P2 ≈ 0.726 atm
To know more about pressure refer to-
https://brainly.com/question/30673967
#SPJ11
Balance the following chemical equation:
As4S6+
02
->
As406+
SO2
What the answer
Answers

Which of the following statements on HPLC modes is true? A. Increasing the polarity of the mobile phase decreases the elution time of polar compounds in normal-phase HPLC B. A non-polar stationary phase is used in normal-phase HPLC C. Compounds have a lower attraction to the mobile phase than to the stationary phase in displacement development D. A polar stationary phase is used in reversed-phase HPLC E. More polar compounds elute first in normal-phase HPLC
Answers
The following statements on HPLC modes are true is more polar compounds elute first in normal-phase HPLC (Option E).
The liquid chromatography (HPLC) is a technique in analytical chemistry employed for the separation, identification, and quantification of elements. It is considered a highly sensitive method, and it works by separating the components in a mixture with the assistance of a solvent under high pressure.
There are two modes of HPLC: Reversed-Phase HPLC (RP-HPLC) and Normal-Phase HPLC (NP-HPLC). In RP-HPLC, a nonpolar stationary phase, such as C18, is used, and polar solvents, such as water, are used as mobile phases. Polar stationary phases, such as silica gel, are used in NP-HPLC, while nonpolar solvents, such as hexane, are used as mobile phases.
More polar compounds have a greater affinity for the polar stationary phase than less polar compounds, which have a higher affinity for the nonpolar mobile phase in NP-HPLC. As a result, less polar compounds elute first in normal-phase HPLC.
Thus, the correct option is E.
Learn more about HPLC: https://brainly.com/question/13490391
#SPJ11
What conclusion did Rutherford draw from his gold-foil experiment? A. Almost all the mass of an atom is concentrated in the nucleus. B. Atoms contain three different subatomic particles. C. Electrons are tiny particles that carry a negative charge. D. The mass of a proton is nearly equal to the mass of a neutron.
Answers
Answer:
A. Almost all of the mass is concentrated in the nucleus.
Explanation:
Because when he shot the alpha particles towards the atoms, most passed through (which meant atom is mostly empty space), but some bounced back and such (which meant mass is concentrated in the nucleus.)
The reason they bounced is because alpha particles are postive and nucleus is positive as well, and we know positives don't attract, rather they repel thus they bounced.
What is the density of tellerium tribromide at 743.5 torr and 23.2 °C
Answers
Answer: The density of tellerium tribromide is 14.8 g/L
Explanation:
The relation between density and Molar mass:
\(d=\frac{PM}{RT}\)
P = pressure of gas = 743.5 torr = 0.98 atm (760torr=1atm)
M = Molar mass = 367 g/mol
R = gas constant =\(0.0821Latm/Kmol\)
T = temperature =\(23.2^0C=(23.2+273)K=296.2K\)
\(n=\frac{PV}{RT}\)
\(d=\frac{0.98atm\times 367g/mol}{0.0820 L atm/K mol\times 296.2K}=14.8g/L\)
The density of tellerium tribromide is 14.8 g/L
During an experiment, solid iodine was placed in a sealed container. The container was gradually heated and purple-colored vapors of iodine formed were observed. Describe this system when it reaches phase equilibrium.
Answers
Answer:
See explanation
Explanation:
A system is said to have attained dynamic equilibrium when the rate of forward reaction and the rate of reverse reaction are equal.
Considering the system under consideration;
I2(s)⇄I2(g)
Heating the container converts solid iodine to purple coloured iodine vapour.
At equilibrium, there will be no net change in the amount of solid iodine and iodine vapour present in the system because the two processes (forward and reverse reactions) occur at the same rate at equilibrium.
The mass number of an element is equal to
Answers
Answer:
The mass number is defined as the total number of protons and neutrons in an atom.
Explanation:
the type of formula that shows the arrangements of atoms and bonds is called
Answers
The type of formula that shows the arrangements of atoms and bonds is called a structural formula.
A structural formula is a representation of a molecule that explicitly shows the connectivity between atoms and the bonds between them. It provides a detailed and visual representation of the molecular structure, indicating how the atoms are bonded and arranged in space.
In a structural formula, the atoms are represented by their chemical symbols, and the bonds between them are shown using lines. The lines represent the shared pairs of electrons that form the bonds. The arrangement of atoms and bonds in the structural formula provides information about the connectivity and spatial orientation of the atoms within the molecule.
Structural formulas are widely used in chemistry to depict the arrangements of atoms and bonds in various compounds, allowing for a better understanding of their chemical properties and behavior.
To know more about structural formula here
https://brainly.com/question/9514179
#SPJ4
--The given question is incomplete, the complete question is
"The type of formula that shows the arrangements of atoms and bonds is called----------------."--
what is the pH of a solution with [H+] = 1.25 x 10^-10M?
Answers
pH = -log[H+]
Given [H+] = 1.25 x 10^-10 M:
pH = -log(1.25 x 10^-10)
pH = -log(1.25) + log(10^-10)
pH ≈ -9 + (-10)
pH ≈ -19
Therefore, the pH of the solution with [H+] = 1.25 x 10^-10 M is approximately -19.
Answer:
9.90
Explanation:
Given [H+] = 1.25 x 10^-10 M, we can calculate the pH using the formula:
pH = -log10([H+])
pH = -log10(1.25 x 10^-10)
Using logarithmic properties:
pH = -log10(1.25) - log10(10^-10)
Since log10(10^-10) is equal to -10:
pH = -log10(1.25) - (-10)
pH = -log10(1.25) + 10
Now, evaluating the logarithm using a calculator:
pH = -0.0969 + 10
pH = 9.9031
Therefore, the pH of the solution with [H+] = 1.25 x 10^-10 M is approximately 9.9031. Rounding it to two decimal places, the pH is approximately 9.90.
1
The process of a solid turning into a liquid is called
Answers
Answer:
Melting
Explanation:
Once heat is added it will turn to a liquid
Melting is the process of turning solid to liquid.
image
The birds in the model above share the same common ancestor. A drought has killed most of the insects in their environment. What is a possible immediate result from this change in the environment?
Answers
Answer:The population of insect eating birds will adapt so they can eat seed to avoid starvation.
Explanation:
trust me-
For a chicken wing.
Part 1: Ligament: Give a description of the Ligament's color, texture, etc. (half a point)
Part 2: Ligament : The Ligament is attached to what tissue? (half a point)
Answers
The ligament of a chicken wing has a white color and a smooth texture.
The ligament is attached to tendon and bones.
What is a Ligament?Ligaments are connective fibrous tissues that forms an attachment from bone to bone. They are white in color and are mostly found in joint surfaces which are attached to bones and tendons.
Ligaments serve to hold bones in place to avoid twisting out of place or snapping easily and support internal organs. In Chicken wings, the ligaments are located at the elbow where it attaches the upper wing to the lower wing.
find out more on Ligaments here: https://brainly.com/question/139369
#SPJ1
what is the cation and anion formula for each compound
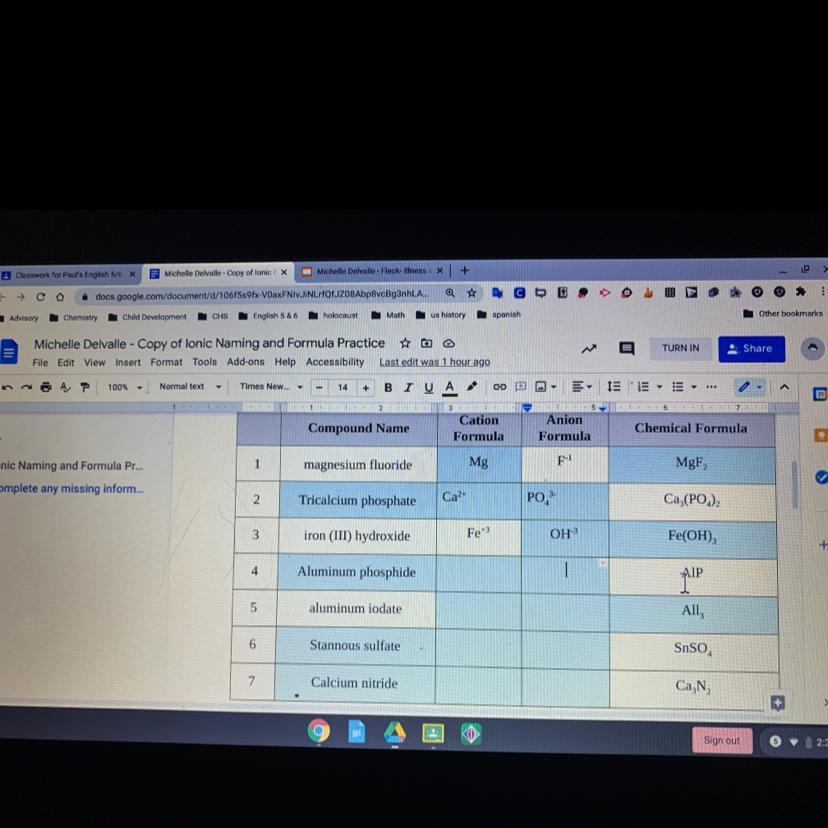
Answers
Answer:
cation ca2+ anion cl-
Explanation:
please see the picture your answer is there


Changes that occur during a chemical reaction are represented by a(n) _?_.
Answers
Answer:
Explanation:
What does it mean by are represented by is it like cells when things can a cell molecule?
____ Al(NO3)3 + ____ FeCl2 --> _____ Fe(NO3)2 + ______AlCl3
~
how to fix/ balance this chemical reaction?
Answers
The balanced equation
2Al(NO₃)₃ + 3FeCl₂ ⇒ 3Fe(NO₃)₂ + 2AlCl₃
Further explanationGiven
Reaction
Al(NO₃)₃ + FeCl₂ --> Fe(NO₃)₂ + AlCl₃
Required
Balanced equation
Solution
Give a coefficient
aAl(NO₃)₃ + bFeCl₂ --> Fe(NO₃)₂ + cAlCl₃
Make an equation
Fe, left=b, right=1⇒b=1
N, left=3a, right=2⇒3a=2⇒a=2/3
Cl, left=2b, right=3c⇒2b=3c⇒2.1=3c⇒c=2/3
The equation becomes :
2/3Al(NO₃)₃ + FeCl₂ --> Fe(NO₃)₂ + 2/3AlCl₃ x3
2Al(NO₃)₃ + 3FeCl₂ --> 3Fe(NO₃)₂ + 2AlCl₃
Find the entropy of 1 mole of N2 molecule consider it as an ideal gas occupying a cubic volume of side 1 cm
choices
1: 74 J/K
2: 37
3:136
4: 62
Answers
The entropy of 1 mole of N2 molecules, considered as an ideal gas occupying a cubic volume of side 1 cm, is approximately 4: 62 J/K.
To find the entropy of 1 mole of N2 molecules, we need to use the formula for entropy:
S = R * ln(W)
Where:
S is the entropy
R is the gas constant
ln is the natural logarithm
W is the number of microstates or ways the system can be arranged
For an ideal gas, the number of microstates can be calculated using the formula:
W = (V^N) / (N!)
Where:
V is the volume
N is the number of molecules
Given that we have 1 mole of N2 molecules, which is approximately 6.022 x 10^23 molecules, and the volume of the cube is 1 cm^3, we can calculate the entropy.
First, let's convert the volume from cm^3 to m^3:
1 cm^3 = (1 x 10^-2 m)^3 = 1 x 10^-6 m^3
Now, we can substitute the values into the formula for W:
W = ((1 x 10^-6 m^3)^(6.022 x 10^23)) / (6.022 x 10^23)!
To simplify the calculation, we can use the fact that ln(W) is equivalent to ln((V^N) / (N!)) = ln(V^N) - ln(N!).
ln(W) = ln((1 x 10^-6 m^3)^(6.022 x 10^23)) - ln(6.022 x 10^23)!
ln(W) = (6.022 x 10^23) * ln(1 x 10^-6 m^3) - ln(6.022 x 10^23)!
Now, substituting the values of ln(1 x 10^-6 m^3) and ln(6.022 x 10^23) into the equation:
ln(W) = (6.022 x 10^23) * (-13.8155) - ln(6.022 x 10^23)!
Finally, we can use the value of the gas constant, R, which is approximately 8.314 J/(mol·K), to calculate the entropy:
S = R * ln(W) = (8.314 J/(mol·K)) * ((6.022 x 10^23) * (-13.8155) - ln(6.022 x 10^23)!)
learn more about entropy
https://brainly.com/question/6364271
#SPJ11
in a circuit, the switch
A) opens and closes the circuit
B) starts and stops the electrons
Answers
Ad by SolarSolution
Paying over $100 for electricity?
Get a free solar panel installation quote with latest incentives and find out how much you could save!
Learn More
2 Answers
Profile photo for William Rose
William Rose
, Electrical Engineer, Mechanic
Answered 1 year ago · Author has 479 answers and 2.2M answer views
Assuming you do mean electronic and not electrical circuits, then the answer is, most of the failures. Powering up and down electronics stresses most components more than leaving them running all the time. First there is temperature stresses as a cold component begins to warm up. Then there are the voltage and current spikes and surges created as capacitors charge up, inductors build their fields, etc. And its not just the components but the stray capacitance and inductance as well.
Basically, when you turn on a device and it doesn’t work, chances are it failed the moment you turned it on (or the moment you last turned it off). They rarely fail while they are running. An old adage in electronics is, the longer a device operates, the longer it will operate.
I always plug in my power supplies for my phone, laptop, etc., before plugging it into the device so the power supply can stabilize without a load on it. Then I plug it into the device. Then I turn on the device. The idea is to minimize the spikes and surges.
BTW, its the same with incandescent light bulbs. A cold filament is rapidly heated which is a thermal stress. Then there is a mechanical stress caused by the current rushing through the coiled filament which creates a mechanical torque on the filament. That’s why you usually (not always) see a bulb fail when you first switch it on
The combustion of 0.1240 kg of propane in the presence of excess oxygen produces 0.3110 kg of carbon dioxide. What is the limiting reactant?
PLEASE I REALLY NEED HELP!!!!
Answers
Answer:
The limiting reactant is the propane gas, C₃H₈ while the percentage yield is 83.77%
Explanation:
Here we have
Propane gas with molecular formula C₃H₈, molar mass = 44.1 g/mol combining with O₂ as follows
C₃H₈ + 5O₂ → 3CO₂ + 4H₂O
Therefore, 1 mole of C₃H₈ combines with 5 moles of O₂ to produce 3 moles CO₂ and 4 moles of H₂O
Mass of propane = 0.1240 kg = 124.0 g
Number of moles of propane = mass of propane/(molar mass of propane)
The number of moles of propane = 124/44.1 = 2.812 moles
The molar mass of CO₂ = 44.01 g/mol
Mass of CO₂ = 0.3110 kg = 311.0 g
Therefore, number of moles of CO₂ = mass of CO₂/(molar mass of CO₂)
The number of moles of CO₂ = 311.0 kg/ 44.01 g/mol = 7.067 moles
Therefore, since 1 mole of propane produces 3 moles of CO₂, 2.812 moles of propane will produce 3 × 2.812 moles or 8.44 moles of CO₂
Therefore;
The limiting reactant is the propane gas, C₃H₈, since the oxygen is in excess
Hence
\(The \ percentage \ yield = \frac{Actual \, yield}{Theoretical \, yield} \times 100 = \frac{7.067}{8.44} \times 100 = 83.77 \%\)
The percentage yield = 83.77%.
Si uno pone un hilo en un hielo y un poco de sal el hilo se levanta el hilo con el hielo. Por que sé relaciona con calor y temperatura, explique con sus propias palabras
Answers
Answer:
Explanation:
El hielo encierra la primavera antes de espolvorear la sal.
Cuando se rocía sal sobre el hielo, disminuye el punto de fusión del hielo 32 ° F a un poco por debajo de 32 °, por lo tanto, se acumula.
A medida que el hielo se vuelve a congelar, encierra la primavera
A 20.0-liter flask contains a mixture of argon at 0.72 atmosphere and oxygen at 1.65 atmospheres. what is the total pressure in the flask? a. 0.93 atm b. 2.37 atm c. 8.44 atm d. 18.6 atm
Answers
A 20.0-liter flask contains a mixture of argon at 0.72 atmosphere and oxygen at 1.65 atmospheres. The total pressure in the flask is 2.37 atm.
The macroscopic characteristics of ideal gases are related by the ideal gas law (PV = nRT). A gas is considered to be perfect if its particles (a) do not interact with one another and (b) occupy no space (have no volume).
The partial pressures of the gases in a mixture of gases are added to determine the total pressure in the flask. This is the partial pressures law of Dalton.
The total pressure of the gases = P1+P2+P3+..Pn=\(P_{total}\)
Therefore, in the given scenario, the flask's total pressure will be equal to 0.72+1.65 = 2.37 atm. Therefore, when a 20.0-liter flask includes a mixture of argon at 0.72 atmospheres and oxygen at 1.65 atmospheres, the total pressure in the flask is 2.37 atm.
Learn more about pressure:
https://brainly.com/question/28012687
#SPJ4
if we react 117 grams of sodium chloride with 340 grams of silver nitrate in a complete reaction what will be the mass, in grams, of the products?
Answers
y’all i have this same question someone please help
in the distillation of a pure material, why does all of the pure material no vaportize once the boiling point is reched.
Answers
In the distillation of a pure material, all of the pure material not vaporize once the boiling point is reached because more heat would need to be added to the distillate in order to vaporize the liquid from its boiling point.
During distillation, the process of vaporizing a liquid and collecting the resulting vapor as a purified substance, it is important to consider the energy requirements involved.
When a liquid reaches its boiling point, it undergoes a phase change from the liquid phase to the gas phase. This phase change requires the input of energy in the form of heat. The heat breaks the intermolecular forces holding the liquid molecules together, allowing them to transition into the gas phase.
The heat required to vaporize a liquid is not solely determined by the boiling point. The heat required to convert a liquid into a gas is known as the heat of vaporization, and it varies depending on the substance.
When distilling a liquid, such as water, the heat of vaporization must be supplied to convert the liquid into vapor. This energy is absorbed by the liquid, and it is essential to provide continuous heating to maintain the distillation process.
As the liquid is heated and reaches its boiling point, vaporization begins. However, the rate at which the liquid vaporizes depends on the amount of heat being supplied. If the heat input is insufficient, the vaporization process will be slower, and not all of the liquid will vaporize at once.
To ensure the complete vaporization of a liquid during distillation, a sufficient amount of heat must be continuously applied to the system. This allows the heat of vaporization to be continually supplied to the liquid, facilitating the conversion of the entire liquid into vapor.
If the heat input is insufficient, the vaporization process will be slower, and the liquid may not vaporize all at once. Providing adequate and continuous heating is crucial to ensure the complete conversion of the liquid into vapor during distillation.
To know more about distillation here
https://brainly.com/question/31829945
#SPJ4
Apply Concepts H2O and H2O2 are binary molecular compounds generally known by their common names, “water” and “hydrogen peroxide.” Following the naming conventions you identified for molecular compounds, what would their names be? Explain your reasoning.
Answers
A compound that consists of two non-metal elements in its structure and compound is called a binary molecular compound. They can have two different elements in their structures bonded by various bonds like carbon dioxide, sodium chloride etc.
The naming of \(\rm H_{2}O\) is dihydrogen oxygen and of \(\rm H_{2}O_{2}\) is dihydrogen dioxygen.
How to name binary molecular compounds?\(\rm H_{2}O\) is a water molecule and is a binary molecular compound as it has one element of oxygen and other elements of hydrogen. The compound has two hydrogen and one oxygen atom and thus will be named dihydrogen oxygen.\(\rm H_{2}O_{2}\) is the peroxide and is a binary molecular compound as it also has hydrogen and oxygen element in its structural formula. The compound has two oxygen and two hydrogen elements in its structure and therefore, will be named dihydrogen dioxygen.Thus, the naming of water is dihydrogen oxygen and hydrogen peroxide is dihydrogen dioxygen.
Learn more about binary molecular compounds here:
https://brainly.com/question/2679241