an organic compound has the formula ch3no. how many valence electrons does this molecule have? a) 10. b) 16. c) 18. d) 22.
Answers
The compound \(CH_{3}NO\) has 18 valence electrons. The correct answer is b) 16.
To determine the number of valence electrons in an organic compound, we can simply add up the valence electrons of each atom in the molecule. The valence electrons for each atom are:
C (carbon) = 4
H (hydrogen) = 1 (3 H atoms = 3)
N (nitrogen) = 5
O (oxygen) = 6
Adding these valence electrons together, we get:
4 + 3 + 5 + 6 = 18
However, we need to subtract the number of bonds in the molecule, which is 4 (1 C-N bond, 3 C-H bonds):
18 - 4 = 14
So the total number of valence electrons in the molecule is 14. However, the question asks for the number of valence electrons in the molecule, not the number of valence electrons in the atoms. Therefore, the correct answer is 16, as there are 2 additional electrons in the C=O double bond.
So the correct answer is b) 16.
Learn more about valence electrons at: https://brainly.com/question/371590
#SPJ11
Related Questions
How many grams of Hydronium chromate are produced when 43.4 g of Tin (IV) chromate combines with35.2 g of Hydronium hydrogen phosphate? Use the following balanced equation:2 (H3O)2HPOA + 1 Sn(CrO4)2 ---> 2 (H30)2CrOA + 1 Sm(HPOA)2

Answers
Answer
Mass of (H30)2CrO = 38 g
Explanation
Given:
Mass of Sn(CrO4)2 = 43.4 g
Mass of (H3O)2HPO4 = 35.2 g
Required: The mass of (H30)2CrO4 that will be produced
Solution:
Calculate the possible mass that could be produced by each reactant, so as to determine the limiting reagent. Use stoichiometry.
For Sn(CrO4)2:
\(\begin{gathered} 43.4\text{ g Sn\lparen CrO}_4\text{\rparen}_2\text{ x }\frac{1\text{ mole Sn\lparen CrO}_4)_2}{350.70\text{ g Sn\lparen CrO}_4)_2}\text{ x }\frac{2\text{ mole \lparen H}_3\text{0\rparen}_2\text{CrO}_4}{1\text{ mole Sn\lparen CrO}_4)_2}\text{ x }\frac{153.9\text{ g \lparen H}_3O)_2CrO_4}{1\text{ mol \lparen H}_3O)_2CrO_4} \\ \\ =\text{ 38 g \lparen H}_3\text{O\rparen}_2\text{CrO}_4 \end{gathered}\)For (H3O)2HPO4
\(\begin{gathered} 35.2\text{ g \lparen H}_3\text{O\rparen}_2\text{HPO}_4\text{ x }\frac{1\text{ mole \lparen H}_3\text{O\rparen}_2\text{HPO}_4\text{ }}{133.97\text{ g }(H_3O)_2HPO_4}\text{ x }\frac{2\text{ mole}}{2\text{ mole}}\text{ x }\frac{153.9\text{ g \lparen H}_3\text{O\rparen}_2\text{CrO}_4}{1\text{ mole \lparen H}_3\text{O\rparen}_2\text{CrO}_4} \\ \\ =\text{ 40.43 g \lparen H}_3\text{O\rparen}_2\text{CrO}_4 \end{gathered}\)Sn(CrO4)2 will produce less (H30)2CrO4 therefore, Sn(CrO4)2 is the limiting reagent.
1. What i the advantage of making unblock lightly non polar? Provide a full explanation of the chemical principle involved
Answers
The advantage of making a solvent unblocking lightly nonpolar is to increase the solubility of polar and ionic compounds.
The solubility of a solute in a solvent is largely determined by the relative polarity of the solute and solvent. Polar solvents dissolve polar solutes, while nonpolar solvents dissolve nonpolar solutes. Making a solvent unblock lightly nonpolar increases its ability to dissolve polar and ionic compounds by reducing the difference in polarity between the solvent and solute.
This allows polar and ionic compounds to be dissolved in a nonpolar solvent, which can be useful in various applications, such as chromatography and chemical reactions.
Learn more about polar and ionic compounds:
https://brainly.com/question/24692494
#SPJ4
The table shows the amount of radioactive element remaining in a sample over a period of time. radioactive decay rate amount of radioactive sample (grams) time (years) 56.0 0 47.1 400 39.6 800 33.3 1,200 28 1,600 part 1: what is the half-life of the element? explain how you determined this. part 2: how long would it take 312 g of the sample to decay to 9.75 grams? show your work or explain your answer.
Answers
1. The half life of the element, given the data from the question is 1600 years
2. The time taken for 312 g of the sample to decay to 9.75 grams is 8000 years
1. How to determine the half life of the elementHalf-life is the time taken for half a material to decay.
To determine the half life of the given element, do the following:
Original amount = 56 gHalf the original amount = 56 / 2 = 28 gTime for 28 g = 1600 yearHalf life of element = 1600 yearsHow to determine the timeWe'll begin by determining the number of half-lives that has elapsed. This can be obtained as follow:
Original amount (N₀) = 312 gAmount remaining (N) = 9.75 gNumber of half-lives (n) =?2ⁿ = N₀ / N
2ⁿ = 312 / 9.75
2ⁿ = 32
2ⁿ = 2⁵
n = 5
Finally, we shall determine the time. This can be obtained as follow:
Half-life (t½) = 1600 yearsNumber of half-lives (n) = 5Time (t) =?t = n × t½
t = 5 × 1600
t = 8000 years
Learn more about half life:
https://brainly.com/question/26374513
#SPJ1
what is the b^2 - 4ac of x^2-5x+4=0
Answers
Answer:
9
Explanation:
Given parameters:
what is b² - 4ac of
Equation x² - 5x + 4 = 0
Solution:
x² - 5x + 4 in the form ax + bx + c = 0
a is the coefficient of x² = 1
b = -5
c = 4
Input the parameters and solve;
b² - 4ac = -5² - 4(1)(4)
= 25 - 16
= 9
1. ni2 (nhbr, ph,o where m, n, and p represent the coefficients in the formula. an analysis of the salt can be obtained if a sample is dissolved by an acid-base reaction in excess acid and then the excess is titrated with naoh. that is, excess hci oh-h20 a 0.185-g sample of the nickel salt was dissolved in 30.00 ml of 0.1013 n hci. the excess hcl required 6.30 ml of 0.1262 n naoh to reach the end point. calculate the weight of the salt that contains one mole of nh3 (that is the equivalent weight of the salt) 2. propose a molecular formula for the salt in 3ni2(nh)mbrn ph20 that is consistent with this experimental equivalent weight.
Answers
The molecular formula for the salt consistent with this experimental equivalent weight is Ni2(NH2)Br(H2O).
What is acid-base reaction?The exchange of one or more hydrogen ions, H +, between species that may be neutral (molecules like water, H 2 O, or acetic acid, CH 3 CO 2 H) or electrically charged (ions like ammonium, NH 4+, hydroxide, OH, or carbonate, CO 32) is known as an acid-base reaction.
The first step to determining the equivalent weight of the salt is to calculate the number of moles of HCl that were used to neutralize the sample. This can be done by using the following formula:
moles HCl = (volume HCl) x (molarity HCl)
moles HCl = (30.00 mL) x (0.1013 mol/L) = 3.04 x 10^-2 mol
Next, we need to calculate the number of moles of NaOH that were used to neutralize the excess HCl. This can be done by using the following formula:
moles NaOH = (volume NaOH) x (molarity NaOH)
moles NaOH = (6.30 mL) x (0.1262 mol/L) = 7.97 x 10^-3 mol
We can use this number to calculate the number of moles of salt that was present in the original sample.
moles salt = (moles HCl) = 7.97 x 10^-3 mol
Now we can use this number to calculate the equivalent weight of the salt. The equivalent weight of a substance is defined as the molar mass of the substance divided by the number of moles of the substance that react with one mole of H+.
Equivalent weight of the salt = (mass of salt) / (moles of salt)
Equivalent weight of the salt = (0.185 g) / (7.97 x 10^-3 mol) = 23.2 g/mol
From the equivalent weight of the salt, we can observe that the equivalent weight is consistent with the formula Ni2 (NH)mBrn(H2O)p (where m,n,p represent coefficients in the formula).
The molar mass of Ni2 (NH)mBrn(H2O)p = 2*(Ni) + m*(NH3) + n*(Br) + p*(H2O)
2*(Ni) + m*(NH3) + n*(Br) + p*(H2O) = 23.2 g/mol
Therefore, the molecular formula for the salt consistent with this experimental equivalent weight is Ni2(NH2)Br(H2O).
To know more about acid-base reaction, visit:
brainly.com/question/15220646
#SPJ4
for problems 24-25: ch4(g) 2 o2(g) ----> co2(g) 2h2o(l) at what rate is ch4 reacting if the rate of water production is 0.082 m/s?
Answers
The rate of CH₄ reacting is 0.041 mol/s.
In this chemical reaction, CH₄(g) reacts with 2 O₂(g) to produce CO₂(g) and 2 H₂O(l). The balanced equation can be written as:
CH₄(g) + 2 O₂(g) → CO₂(g) + 2 H₂O(l).
To find the rate of CH₄ reaction when the rate of water production is 0.082 mol/s, we can use the stoichiometry of the equation.
Since 2 moles of water are produced for every 1 mole of CH₄ consumed, we can set up the following proportion:
Rate of CH₄ reaction / Rate of H₂O production = 1 mole CH₄ / 2 moles H₂O
Now, substitute the given rate of water production:
Rate of CH₄ reaction / 0.082 mol/s = 1 / 2
Solving for the rate of CH₄ reaction:
Rate of CH₄ reaction = 0.082 mol/s × (1/2) = 0.041 mol/s
Learn more about chemical reaction at https://brainly.com/question/29762391
#SPJ11
Determine the molarity of a solution that contains 30 moles naoh in 0.80 liters of solution?
Answers
Taking into account the definition of molarity, the molarity of a solution that contains 30 moles naoh in 0.80 liters of solution is 37.5 \(\frac{moles}{liters}\).
Definition of molarityMolar concentration or molarity is a measure of the concentration of a solute in a solution and indicates the number of moles of solute that are dissolved in a given volume.
The molarity of a solution is calculated by dividing the moles of solute by the volume of the solution:
\(Molarity=\frac{number of moles}{volume}\)
Molarity is expressed in units \(\frac{moles}{liters}\).
This caseIn this case, you have:
number of moles= 30 molesvolume= 0.80 LReplacing in the definition of molarity:
\(Molarity=\frac{30 moles }{0.80 L}\)
Solving:
Molarity= 37.5 \(\frac{moles}{liters}\)
Finally, the molarity of a solution that contains 30 moles naoh in 0.80 liters of solution is 37.5 \(\frac{moles}{liters}\).
Learn more about molarity:
brainly.com/question/9324116
brainly.com/question/10608366
brainly.com/question/7429224
#SPJ1
In steps 3 and 4 of the citric acid cycle, successive oxidations produce ________ as a waste product and the four-carbon carrier ________. In steps 3 and 4 of the citric acid cycle, successive oxidations produce ________ as a waste product and the four-carbon carrier ________. CO2; malate CO2; succinyl-SCoA ADP; oxaloacetate ADP; succinyl-SCoA CO2; oxaloacetate
Answers
Answer:
CO2; succinyl-SCoA
Explanation:
Citric acid cycle, also called Krebs cycle is the second stage of aerobic cellular respiration, which occurs in the mitochondrion. The kreb's cycle is made up of 7 distinct steps, with each involving series of enzymatic reactions.
According to this question, step 3 and 4 of the citric acid cycle involves an oxidation and decarboxylation reaction to produce 2 molecules of carbon dioxide (CO2) and a 4-carbon compound called succinyl group. A coenzyme A binds to the succinyl group to form succinyl-SCoA.
Please help
urgently!

Answers
1. We can see here that energy is required to change the phase of matter. For example, energy is required to melt ice, vaporize water, and condense steam. The amount of energy required to change the phase of matter is called the latent heat.
What is energy?Energy is a fundamental concept in physics and refers to the ability or capacity of a system to do work or produce a change.
2. The demonstration on the sample of water showed that water can exist in three phases: solid, liquid, and gas. The solid phase is ice, the liquid phase is water, and the gas phase is steam.
The demonstration started with ice at 0°C. As heat was added to the ice, the temperature of the ice increased. However, the ice did not melt until the temperature reached 0°C. This is because the energy from the heat was used to break the bonds between the water molecules in the ice. Once the bonds were broken, the ice melted and became water.
3. Completing the
When all the intermolecular bonds are overcome, the transition between phases is complete. The energy of any substance includes the kinetic energy, potential energy, and thermal energy of its particles.Page 4:
Heating and cooling curves are graphical representations of how temperature changes during the process of heating or cooling a substance. They illustrate the relationship between temperature and the state of matter.
Heating curves represent the temperature changes of a substance as it is heated.Cooling curves, on the other hand, represent the temperature changes of a substance as it is cooled.Both curves show:
Plateaus or flat sections: These occur during phase transitions where the temperature remains constant despite the addition or removal of heat.Changes in slope: The slope of the curve represents the rate of temperature change. Steeper slopes indicate faster changes in temperature, while shallower slopes indicate slower changes.Learn more about energy on https://brainly.com/question/2003548
#SPJ1
The interaction of aligned sp3 orbitals on adjacent atoms is _____________
It will ____________ (increase/decrease) the internal energy of a rotamer.
Answers
Answer: The interaction of aligned sp3 orbitals on adjacent atoms is called sigma orbitals. It can increase the internal energy of a rotamer.
Explanation:
The interaction of aligned sp3 orbitals on adjacent atoms is called σ-bonding. It occurs due to axial overlapping of sp3 orbitals.This interaction can increase the internal energy of a rotamer.
suppose you are performing a hydroboration oxidation of 1-hexene to form 1-hexanol. your reaction includes 0.84 ml of 1-hexene, which has a density of 0.673 g/ml, 1.73 ml of 1.00 m borane in thf, and 1.26 ml of 30.0% m/v hydrogen peroxide. what is the theoretical yield of 1-hexanol (in g) for the reaction?
Answers
The theoretical yield of 1-hexanol (in g) for the reaction is: 102.18 g
Theoretical Yield of 1-HexanolIn the reaction for the hydroboration oxidation of 1-hexene to form 1-hexanol, the theoretical yield of 1-hexanol (in g) can be calculated by applying the formula:
Theoretical yield = (mass of limiting reactant x molecular weight of 1-hexanol) / molecular weight of limiting reactant
Given that the reaction includes 0.84 ml of 1-hexene, which has a density of 0.673 g/ml, 1.73 ml of 1.00 m borane in thf, and 1.26 ml of 30.0% m/v hydrogen peroxide. The molecular weight of 1-hexene is 84.16 g/mol, and the molecular weight of 1-hexanol is 102.18 g/mol.
To find out the limiting reactant, we need to first calculate the moles of each reactant as follows:
Moles of 1-hexene = volume x density / molecular weight = 0.84 ml x 0.673 g/ml / 84.16 g/mol = 0.00675 mol
Moles of borane = volume x concentration = 1.73 ml x 1.00 M = 0.00173 mol
Moles of hydrogen peroxide = volume x concentration = 1.26 ml x 30.0% m/v / (100 g/L) / (34.0147 g/mol) = 0.00113 mol
From the above calculations, the borane is the limiting reactant since it produces the least amount of moles of product. Therefore, the moles of 1-hexanol produced is also equal to the moles of borane used in the reaction.
Moles of 1-hexanol produced = moles of borane used = 0.00173 mol
The theoretical yield of 1-hexanol can be calculated by applying the formula:
Theoretical yield = (mass of limiting reactant x molecular weight of 1-hexanol) / molecular weight of limiting reactant= (0.00173 mol x 102.18 g/mol) / 0.00173 mol= 102.18 g
The theoretical yield of 1-hexanol is 102.18 g.
To know more about theoretical Yield refer here:
https://brainly.com/question/12704041#
#SPJ11
(c) A new car produces 132 g of carbon dioxide per kilometre travelled.
Petrol contains mainly octane, C8H₁8. This is the equation for the complete combustion
of octane.
C8H18 +12.5 O₂8CO2 +9H₂0
Calculate the mass of octane that burns to produce 132 g of carbon dioxide.
[3]
Answers
132 gm of CO₂ is generated by combustion of 342 gm of C₈H₁₈.
What is Molecular Mass?The molar mass of a chemical compound is defined as the ratio between the mass and the amount of that substance in any sample. A material's molar mass is a bulk characteristic rather than a molecular one.
The molar mass of a substance is its mass expressed in grams per mole. G/mol, or grams per mole, is the sign for molar mass. The ratio between the mass of an isotope and the mass of the isotope carbon is known as the isotopic atomic mass, or mass of a single isotope of any particular element. -12
The reaction is :
C₈H₁₈ + 12.5O₂ ----> 8CO₂ + 9H₂O
Molar mass of C₈H₁₈ is = 12*8 + 18*1 = 114 gm
Molar mass of CO₂ is = 12+ 16*2 = 44gm
44gm of CO₂ is generated by 114 gm of C₈H₁₈
1 gm of CO₂ is generated by 114/44 gm of C₈H₁₈
132 gm of CO₂ is generated by combustion of 114*132/44 = 342 gm of C₈H₁₈
To learn more about Molar mass refer to :
brainly.com/question/24172406
#SPJ1
Nutritionists express energy in calories, which are in fact 1000 real calories. one real calorie is equivalent to 4.18 joules, the universal energy unit. thus, one nutrition calorie is equivalent to:______
Answers
Nutritionists express energy in calories, which are 1000 real calories. One real calorie is equivalent to 4.18 joules, the universal energy unit and hence, one nutrition calorie is equivalent to: 4.18 kilojoules.
What is nutrition calorie?In the nutrition world, calorie indicate amount of energy in foods. As a food calorie is equal to 1,000 small calories, it is also called a large calorie or a kilocalorie (kcal). Food calories come from these four sources: carbohydrates, proteins, fats, and alcohol.
The calorie and nutrient content of single ingredients and individual foods is found in the USDA's National Nutrient Database. Most of the packaged foods list information in the Nutrition Facts panel.
To know more about nutrition calorie, refer
https://brainly.com/question/8070307
#SPJ4
Can you please help resolve and explain?
Answers
The Ka of an acid indicates how strong this acid is. This means that greater acidity constant Ka corresponds to stronger acids, and lower Ka corresponds to weakest acids. I the example the acetic acid (CH3COOH) has an acidity constant 6 order of magnitude greater than the HCO3- there fore acetic acid is stronger than HCO3-
Chemists can identify the composition of some unknown salts by conducting a flame test. When potassium salts are heated in a flame, a purple color is observed.
This is due to the movement of electrons between energy levels. What is the electron configuration of a potassium atom at ground state?
answer choices
1s2; 2s2; 2p6; 3s2; 3p6; 4d1
1s2; 2s2; 2p6; 3s2;3p6; 3d1
1s2; 2s2; 2d6; 3s2; 3d6; 4s1
1s2; 2s2; 2p6; 3s2; 3p6; 4s1
Answers
The electron configuration of a potassium atom at ground state is 1s²2s²2p⁶3s²3p⁶4s¹. Therefore, option D is correct.
What is an electronic configuration?The electron configuration of an element can be explained as electrons being occupied in different energy levels of an atom of a specific element. In the electron configuration, the electrons are usually written as a superscript of atomic subshells. For example, the electron configuration of Helium can be represented as 1s²2s².
The sequence of completely filled subshells similar to neighboring the electronic configuration of a noble gas is represented by square brackets. The principal quantum number (n) will be used to denote the maximum number of electrons in an electron shell.
The total number of electrons occupied in the given electronic configuration 1s²2s²2p⁶3s²3p⁶4s¹ is 19. The atomic number of potassium is 19 therefore it is the configuration of potassium.
Learn more about electronic configuration, here:
brainly.com/question/5624100
#SPJ4
which of the following compounds can exhibit linkage isomerism? a. [ni(nh3)6]2 b. [cr(co)3(nh3)3]3 c. [cr(h2o)4br2] d. [fe(co)5ono]2 e. [cr(h2o)4br2]
Answers
Linkage isomerism is a type of isomerism exhibited by coordination compounds where the ligand can bind to the central metal ion through different atoms.
Out of the given options, compounds c and e can exhibit linkage isomerism as they contain the same ligands, i.e., four water molecules and two bromide ions, but the bromide ions can bind to the central chromium ion either through the two different bromine atoms.
In contrast, the other compounds do not contain such ligands that can show linkage isomerism.
[Ni(NH3)6]2 contains only one type of ligand, i.e., NH3.
Similarly, [Cr(CO)3(NH3)3]3, and [Fe(CO)5ONO]2 also have a single type of ligand attached to the metal ion. Therefore, compounds c and e are the only compounds that can exhibit linkage isomerism out of the given options.
To know more about isomerism. please visit.....
brainly.com/question/17569347
#SPJ11
What is the poh of a solution with a ph of 1.30? PLZZ ANSWER
Answers
Answer:
12.70
Explanation:
How did the philosopher's stone affect alchemy?
Answers
Alchemists thought the philosopher's stone had the power to change base metals like lead into precious metals like gold or silver and could be utilized as an elixir of life to extend life.
What is a metal?Any of a wide range of opaque, fusible, ductile, & usually glossy substances, especially, one that is a chemical substance as opposed to an alloy. These substances are good conductors of heat and electricity create cations by the losses of electrons, or produce basic oxides and hydroxides.
How is metal made?Mass metal making, sheet metal formation, & sheet-bulk metal forming are the three different types of metal forming techniques. Forging, rolling, deep drawing, extrusion, wire drawing, & bending are just few common methods of metal formation.
To know more about metal visit :
https://brainly.com/question/4701542
#SPJ1
Why is copper a good conductor of electricity?
A.
Copper atoms hold onto their electrons very tightly.
B.
Electrons can move easily between copper atoms.
C.
Copper atoms are bonded together in molecules.
D.
Copper atoms can flow quickly along a wire.
Answers
Answer:
B. Electrons can move easily between copper atoms.
Explanation:
N2O4 ⇌ 2NO2
for the following reaction at 373 K, Kc = 0.36. If initial concentration of N2O4 is 0.1 mol dm^-3, what is the equilibrium concentration of NO2? (Is there a way to solve this without using quadratics?)
Answers
Okay, let's solve this step-by-step without using quadratics:
1) The equilibrium constant Kc = 0.36 means the equilibrium lies to the left. So there will be more N2O4 than NO2 at equilibrium.
2) The initial concentration of N2O4 is 0.1 mol dm^-3. Let's call this [N2O4]initial.
3) At equilibrium, the concentrations of N2O4 and NO2 will be [N2O4]equil and [NO2]equil respectively.
4) We know the equilibrium constant expression for this reaction is:
Kc = ([NO2]equil)^2 / [N2O4]equil
5) Setting this equal to 0.36 and plugging in 0.1 for [N2O4]initial, we get:
0.36 = ([NO2]equil)^2 / (0.1 - [NO2]equil)
6) Simplifying, we get:
0.036 = [NO2]equil^2
7) Taking the square root of both sides, we get:
[NO2]equil = 0.06 mol dm^-3
So the equilibrium concentration of NO2 is 0.06 mol dm^-3.
Let me know if you have any other questions! I can also provide a more step-by-step explanation if needed.
Explain the charge, the location and the size of protons, and electron.
Answers
Protons are positively charged particles that are located inside the nucleus at the center of the atom. Protons have a mass of 1 amu or atomic mass unit.
Electrons are negatively charged particles found outside the nucleus. Electrons have a mass of approximately 0 amu or atomic mass unit.
hope it helps :)
please mark brainliest!!!
giving brainly if correct

Answers
Answer:
The corect answer would be C.
Explanation:
The flow rate set at a differentt time would be the correct measurement beecause wats and speed add up to your main answer.
The rate of flow is basically the speed, and when you measure the speed of a wagon, you don’t wait for the entire vehicle and the trailer to get over the finish line before you stop the timer.
Enter your answer in the provided box. Calculate the wavelength of a
photon of electromagnetic radiation with a frequency of 61.7 MHz. m
Be sure to answer all parts. Calculate the energy of a photon of
electromagnetic radiation with a wavelength of 582.8 nm. * 10 Report
your answer in scientific notation using the provided boxes.
Answers
we find the energy to be approximately \(3.41 * 10^-19\) Joules is the answer.
To calculate the wavelength of a photon with a frequency of 61.7 MHz, we can use the formula: wavelength = speed of light / frequency. The speed of light is approximately\(3 * 10^8\) meters per second.
Converting the frequency to Hz (\(1 MHz = 10^6 Hz\)), we have \(61.7 * 10^6\)Hz.
Plugging these values into the formula, we get: wavelength =\((3 * 10^8 m/s) / (61.7 * 10^6 Hz).\)
Simplifying, we find the wavelength to be approximately 4.862 meters.
Now, to calculate the energy of a photon with a wavelength of 582.8 nm, we can use the equation: energy = Planck's constant × speed of light / wavelength.
Planck's constant is approximately \(6.63 * 10^-34\) Joule-seconds.
Converting the wavelength to meters (\(1 nm = 10^-9 m\)), we have \(582.8 * 10^-9 m.\)
Plugging these values into the equation, we get: energy =\((6.63 * 10^-34J·s) * (3 * 10^8 m/s) / (582.8 * 10^-9 m).\)
Simplifying, we find the energy to be approximately \(3.41 * 10^-19\) Joules.
know more about wavelength
https://brainly.com/question/31143857
#SPJ11
Two spheres are measured and are both found to have a mass of 24 grams.If sphere A has a density of 12 g/mL and sphere B has a density of 3 g/mL.What can you conclude about the volume of each?
Answers
Answer:
Volume of sphere A = 2 mL
Volume of sphere B = 8 mL
The volume of sphere B is 4 times the volume of sphere A.
Explanation:
We'll begin by calculating the volume of each sphere.
Data obtained from the question include:
Sphere A:
Mass = 24 g
Density = 12 g/mL
Volume =?
Sphere B:
Mass = 24 g
Density = 3 g/mL
Volume =?
Next, we shall determine the volume of each sphere. This can be obtained as follow:
For sphere A:
Density = mass /volume
12 = 24/volume
Cross multiply
12 × volume = 24
Divide both side by 12
Volume = 24/12
Volume = 2 mL
For sphere B:
Density = mass /volume
3 = 24/volume
Cross multiply
3 × volume = 24
Divide both side by 3
Volume = 24/3
Volume = 8 mL
Summary:
Volume of sphere A = 2 mL
Volume of sphere B = 8 mL
Comparing the volume of both sphere, we have:
Va /Vb = 2/8
Va /Vb = 1/4
Cross multiply
Va × 4 = Vb
Vb = 4Va
From the above illustration,
The volume of sphere B is 4 times the volume of sphere A.
It has been concluded that the volume of sphere A has been 4 times greater than the volume of sphere B.
Density can be defined as the mass per unit volume. Density can be expressed as:
Density = \(\rm \dfrac{Mass}{Volume}\)
Sphere A has a mass of 24 grams, and a density of 12 g/ml.
Sphere B has a mass of 24 grams and a density of 3 g/ml.
Since the spheres have the same mass, the relationship can be given as:
Density of A × Volume of A = Density of B × Volume of B
12 × Volume of A = 3 × Volume of B
4 × Volume of A = Volume of B
Thus, it has been concluded that the volume of sphere A has been 4 times greater than the volume of sphere B.
For more information about the volume, refer to the link:
https://brainly.com/question/16924154
How many grams of Al will be deposited from molten AlCl3 by a current of 15.0 amp flowing for 24.0 hr ( 1 Faraday = 96500 C)
0.538 g
0.0335 g
363 g
121 g
Answers
Answer:
121 aprox.
Explanation:
mass/molar mass = Q/CF
where mass=?
molar mass of Al=27
Q=It = 15×24×60×60
C=3
F=96500
Determine the pressure change when a constant volume of gas with an initial pressure of 10 am is cooled from 40 degrees Celsius to 20 degrees Celsius.
Answers
Answer:
To determine the pressure change when a constant volume of gas is cooled from 40°C to 20°C, we can use the ideal gas law, which relates the pressure, volume, temperature, and number of moles of a gas:
PV = nRT
where P is the pressure, V is the volume, n is the number of moles, R is the ideal gas constant, and T is the temperature in Kelvin.
Since we are dealing with a constant volume of gas, we can simplify the ideal gas law to:
P1/T1 = P2/T2
where P1 and T1 are the initial pressure and temperature, and P2 and T2 are the final pressure and temperature.
Converting the temperatures to Kelvin:
T1 = 40°C + 273.15 = 313.15 K
T2 = 20°C + 273.15 = 293.15 K
Substituting the values into the equation:
10 atm / 313.15 K = P2 / 293.15 K
Solving for P2:
P2 = (10 atm / 313.15 K) x 293.15 K
P2 = 9.354 atm
Therefore, the pressure of the gas decreases from 10 atm to 9.354 atm when cooled from 40°C to 20°C at constant volume.
An aluminum cube of mass mAl = 44.25 g and temperature TAl = 22.3°C, is immersed in a Styrofoam cup filled with liquid nitrogen (LN2), which has a temperature TLN2 = -195.8° C. Heat will transfer from the cube to the LN2, which will cause LN2 to evaporate. After 120 seconds, 34.50 g of LN2 has evaporated (mLN2 = 34.50 g).Calculate the latent heat of vaporization Lv of LN2.
2) Calculate the rate of heat flow R (calories/second) at which heat from the cube transfers into the LN2, causing it to evaporate. Use your calculated value of Lv from question 1. Provide the answer (R) in units of calories and seconds, with two digits after the decimal point.
Answers
The value of L v is approximately 15.82 calories/gram (rounded to two decimal places).
To calculate the latent heat of vaporization ( L v) of LN2, we can use the formula:
L v = Q / mLN2
where:
Q = heat transferred
mLN2 = mass of LN2 evaporated
We need to calculate Q first. The heat transferred is equal to the heat lost by the aluminum cube. We can calculate Q using the equation:
Q = mAl × CAl × ΔTAl
where:
mAl = mass of aluminum cube
CAl = specific heat capacity of aluminum
ΔTAl = change in temperature of aluminum cube
Given:
mAl = 44.25 g
TAl = 22.3°C
TLN2 = -195.8°C
mLN2 = 34.50 g
The specific heat capacity of aluminum (CAl) is approximately 0.897 J/g°C.
First, let's calculate ΔTAl:
ΔTAl = TLN2 - TAl
ΔTAl = (-195.8 - 22.3)°C
ΔTAl = -218.1°C
Next, let's convert ΔTAl from Celsius to Kelvin:
ΔTAl(K) = ΔTAl + 273.15
ΔTAl(K) = -218.1 + 273.15
ΔTAl(K) = 55.05 K
Now, we can calculate Q:
Q = mAl × CAl × ΔTAl(K)
Q = 44.25 g × 0.897 J/g°C ×55.05 K
Q = 2197.05 J
Now, we can calculate L v:
L v = Q / mLN2
L v = 2197.05 J / 34.50 g
To convert L v to calories and grams, we can use the conversion factors:
1 calorie = 4.184 J
1 gram = 0.001 kg
L v calories = L v × (1 calorie / 4.184 J)
L v grams = mLN2 × (1 g / 0.001 kg)
Finally, let's calculate L v in calories and grams:
L v calories = (2197.05 J / 34.50 g) × (1 calorie / 4.184 J) ≈ 15.82 calories (rounded to two decimal places)
L v grams = 34.50 g × (1 g / 0.001 kg) = 34,500 grams
Therefore, the value of L v is approximately 15.82 calories/gram (rounded to two decimal places).
To know more about vaporization:
https://brainly.com/question/30762921
#SPJ4
A gas is collected at 20.0 °C and 725.0 mm Hg. When the temperature is
changed to 0 °C, what is the resulting pressure?
Answers
Answer:
676mmHg
Explanation:
Using the formula;
P1/T1 = P2/T2
Where;
P1 = initial pressure (mmHg)
P2 = final pressure (mmHg)
T1 = initial temperature (K)
T2 = final temperature (K)
According to the information provided in this question;
P1 = 725.0mmHg
P2 = ?
T1 = 20°C = 20 + 273 = 293K
T2 = 0°C = 0 + 273 = 273K
Using P1/T1 = P2/T2
725/293 = P2/273
Cross multiply
725 × 273 = 293 × P2
197925 = 293P2
P2 = 197925 ÷ 293
P2 = 676mmHg.
The resulting pressure is 676mmHg
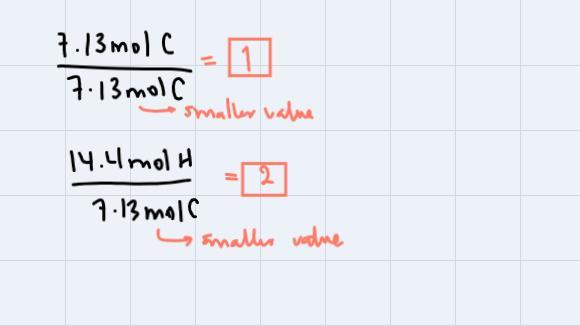
What is the molecular formula of the compound with a molecular weight of 112 g/mol and percent composition: 85.6% C and 14.4% H?C8H16CH2C4H8C2H4
Answers
To find the molecular formula of this compound, what we're going to do is to follow up the steps:
Step 1: Pass all the percentages to grams. This is, just to change the unit:
Step 2: Divide each mass by respective molar mass to obtain the moles of each element:
Step 3: Divide all the amounts in moles through by smaller value obtained:
These are the subscripts for C and H respectively. Thus our empirical formula is CH2. We're asked to find the molecular formula so, we could use the fact that the compound has a molecular weight of 112 g/mol. If we analyze, CH2 has a molecular weight of 14g/mol, so:
Step 4: We're going to divide the molecular weight of the compound with molecular formula through by the molecular weight of the compound with empirical formula:
This means that our molecular formula will be eight times the subscripts of the empirical formula. Therefore, the answer is:




In an electrochemical cell, equilibrium is the point at which:
A. None of these
B. both electrodes have an equal number of electrons.
C. ion concentrations are no longer changing and the voltage is 0.
D. Equilibrium cannot be reached in a voltaic cell because electrons only flow in one direction.
Answers
Answer:
C. ion concentrations are no longer changing and the voltage is 0.
Explanation:
Electrochemical cells are a device that generates current from redox reactions. In the cell, the equilibrium is reached when the voltage drops to 0. Thus, option C is correct.
What is an electrochemical cell?An electrochemical cell is a voltaic device that uses chemical energy to generate electrical energy and vice versa. It is of two types electrolytic and galvanic cells depending on the energy it produces.
The electrochemical cell comprises two electrodes namely an anode and cathode, a salt bridge or the porous barrier, and an external connection.
The connection shows the voltage that indicates the equilibrium. When the current flow stops and the voltage reaches zero, the electrochemical cell is said to be in equilibrium.
Therefore, no change in the ionic concentrations and zero voltage indicates equilibrium.
Learn more about electrochemical cells here:
https://brainly.com/question/16790905
#SPJ5