A triangle has vertices with coordinates (2,0), (3, -1) and (-2,-5). If the triangle is dilated by a scale factor of 3 with the origin as the center of dilation, what are the coordinates of the vertices of the image?
a
(5,3), (6,2), (1,-2)
b
(6,0), (9,-3), (-6,-15)
c
(2/3,0), (1,-1/3), (-2/3,-5/3)
d
(-1,-3), (0,-4), (-5,-8)
Answers
Related Questions
The relative atomic mass of hydrogen is 1, and that of carbon is 12.
12 g of an unknown hydrocarbon (containing hydrogen and carbon only) was found to contain 9 g of carbon. This is shown below. What is the value of x?
Answers
Answer:
The value of x in the hydrocarbon formula CxHy is 1.
Explanation:
Here's how to arrive at the solution:
Since the relative atomic mass of carbon is 12 and the hydrocarbon contains 9g of carbon, we know that there are 9/12 = 0.75 moles of carbon in the hydrocarbon.
Since the hydrocarbon only contains hydrogen and carbon, the remaining mass (12g - 9g = 3g) must be due to hydrogen.
To find the number of moles of hydrogen, we need to convert the mass of hydrogen to moles. The relative atomic mass of hydrogen is 1, so the number of moles of hydrogen is 3/1 = 3 moles.
Therefore, the ratio of carbon to hydrogen in the hydrocarbon is 0.75 moles of carbon to 3 moles of hydrogen, which simplifies to 1:4.
The general formula for the hydrocarbon can be written as CxHy. Since the ratio of carbon to hydrogen is 1:4, we know that x = 1 and y = 4. Thanks.
20 points PLS HURRY
What will most likely occur if communities around the world increase the amount of wood they burn for heat?
A CO2 in the atmosphere will increase.
В CO2 in the atmosphere will decrease.
C Dissolved oxygen in the water will increase.
D Dissolved oxygen in the water will decrease.
Answers
Answer:
the answer is a shh /..\ yall smell that
Explanation:
which of the following represents a rate law for the overall reaction that is consistent with the proposed mechanism? responses rate Rate= k[H2][ICl]2Rate= k[H2][ICl] Rate= k[H2][HI][ICl]2
Answers
The proposed mechanism indicates that the overall reaction is a two-step process. In the first step, H2 reacts with ICl to form HCl and I2. In the second step, HCl and HI react to form H2 and ICl.
Therefore, the rate law for the overall reaction should be expressed as:
Rate= k[H2][HI][ICl]2 where k is the rate constant of the reaction and [H2], [HI], and [ICl] represent the concentrations of the reactants. The rate law indicates that the rate of the overall reaction is directly proportional to the concentration of the three reactants and is proportional to the square of the concentration of ICl. This is because, in the second step, two molecules of ICl are consumed to form H2 and HI.
Therefore, the rate law for the overall reaction is Rate= k[H2][HI][ICl]2, which is consistent with the proposed mechanism.
Learn more about reactants
brainly.com/question/17096236
#SPJ4
Once balanced, what is the coefficient of HCl in the following reaction:
Mg + HCl → MgCl2 + H2
Group of answer choices
A. 1
B. 2
C. 0
D. 3
Answers
Answer:
B. 2
I hope this helps.
Explanation:
Mg + 2HCl → MgCl2 + H2
Given the following equation: Mg + 2HCI → MgCl₂ + H₂
How many moles of H₂ can be produced by reacting 2 moles
of HCI?
Answers
Taking into account the reaction stoichiometry, 1 mole of H₂ can be produced by reacting 2 moles of HCI.
Reaction stoichiometryIn first place, the balanced reaction is:
Mg + 2 HCl → MgCl₂ + H₂
By reaction stoichiometry (that is, the relationship between the amount of reagents and products in a chemical reaction), the following amounts of moles of each compound participate in the reaction:
Mg: 1 moleHCl: 2 molesMgCl₂: 1 moleH₂: 1 moleMoles of H₂ producedBy reaction stoichiometry 2 moles of HCl form 1 mole of H₂.
Learn more about the reaction stoichiometry:
brainly.com/question/24741074
brainly.com/question/24653699
#SPJ1
How many milliliters of a 25% (m/v) NaOH solution would contain 75 g of NaOH?
A 19 mL
B) 25 mL
C
33 mL
D
75 mL
E
3.0 x 102 mL
Answers
Answer:
E 3.0 x 10² mL.
Explanation:
Hello there!
In this case, according to the formula for the calculation of the mass-volume percent:
\(\% m/V=\frac{m_{solute}}{V_{solution}}*100\%\)
Whereas it is necessary to know the mass of the solute and the volume of the solution. Thus, given the mass of NaOH as the solute, the volume of the solution would be:
\(V_{solution}=\frac{m_{solute}}{\% m/V}*100\%\)
Then, by plugging in we obtain:
\(V_{solution}=\frac{75g}{25\%}*100\%\\\\V_{solution}=3.0x10^2mL\)
Thus, the answer is E 3.0 x 10² mL.
Best regards!
It is difficult to break the ionic bonds in a compound because of the
Answers
Draw a structural formula for the major product of the reaction shown.
Answers
Draw a structural formula for the major product of the reaction shown:
The structural formula for the major product (2-butene) of the given reaction is as follows:$$\ce{CH3CH2CH=CH2}$$
The given reaction is an acid-catalyzed dehydration reaction.
During the reaction, the hydroxyl group (OH) and the adjacent hydrogen atoms (H) on the reactant alcohol (2-butanol) undergo dehydration (loss of water) to form an alkene (2-butene) as the major product.
The reaction is shown below:$$\ce{CH3CH2CH2CH2OH + H2SO4 ->[\Delta] CH3CH2CH=CH2 + H2O}$$To draw the structural formula for the major product of the given reaction, we need to consider the following points:
1. The reactant alcohol (2-butanol) is a four-carbon alcohol with the hydroxyl group (OH) attached to the second carbon atom (C2) of the chain.
2. The product alkene (2-butene) will be a four-carbon alkene with a double bond between the second and third carbon atoms (C2 and C3) of the chain.
The other two carbon atoms will have a single bond with the adjacent carbon atoms and a hydrogen atom each attached to them.
3. The major product will be formed via the elimination of water (dehydration) between the hydroxyl group (OH) and the adjacent hydrogen atoms (H) on the second carbon atom (C2) of the reactant alcohol (2-butanol).
4. The acid catalyst (H2SO4) does not participate in the reaction and remains unchanged. It only facilitates the formation of the alkene by providing a proton (H+) to the hydroxyl group (OH) and a medium for the elimination of water.
For more such questions on alkene
https://brainly.com/question/27704061
#SPJ8
26. What was the earliest energy source for humans?
natural gas
oil
coal
wood
All changes saved
Answers
The earliest energy source for humans was wood. Wood has been used as a primary energy source for humans since prehistoric times, making it the earliest known source of energy.
Early humans used wood to create fire, which was used for warmth, cooking, and light. As human societies developed, the use of wood expanded to include building materials, transportation, and other forms of energy production, such as charcoal.
While wood has been a reliable source of energy for humans throughout history, the widespread use of fossil fuels in the industrial era led to a decline in the use of wood as a primary energy source.
However, wood still plays an important role in many parts of the world, particularly in developing countries where it is used for cooking and heating. Additionally, the use of wood for energy is gaining renewed interest as a form of renewable energy and an alternative to fossil fuels.
Learn more about wood here:
https://brainly.com/question/10967023
#SPJ1
Please help this affects my grade a lot and im confused
Double Replacement/Combustion I need to find the product of each one
zinc oxide + Cobalt(III) nitrite
Calcium hydroxide + phosphoric acid
Copper(I) dichromate + Iron(III) arsenate
Hydrochloric acid + aluminum hydroxide
Answers
1 ) Cobalt(II) nitrate react with nitrate zinc(II) to produce cobalt(III)-zinc oxide, nitrogen dioxide and oxygen
2Co(NO3)2 + Zn(NO3)2 → Co2ZnO4 + 6NO2 + O2
2) Calcium hydroxide react with phosphoric acid to produce calcium, hydrogen phosphate and water
Ca(OH)2 + H3PO4 → CaHPO4 + 2H2O
3) Copper(II) dichromate will react with Iron(III) arsenate to form Copper(II) Arsenate and Iron(III) dichromate
3Cr2CuO7 + 2AsFeO4 → Cu3(AsO4)2 + Fe2(Cr2O7)3
Reaction Type : Double Displacement
4) aluminium hydroxide and hydrochloric acid react to form aluminium chloride and water
Al(OH)3 + 3HCl → AlCl3 + 3H2O
Which letter indicates the asthenosphere
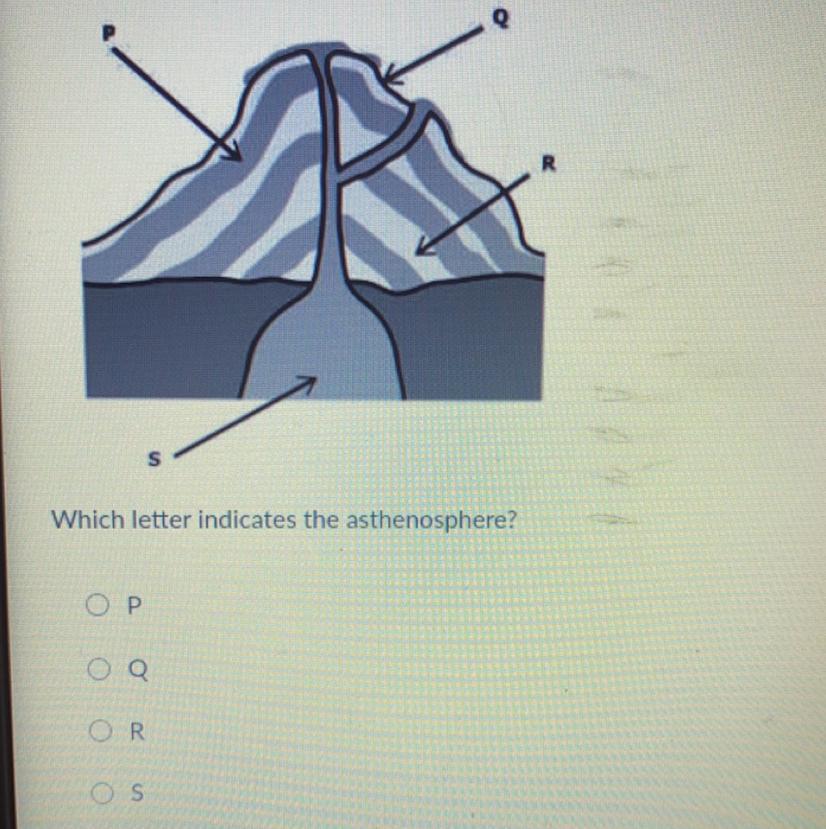
Answers
Answer:
q
Explanation:
Suppose a solution has a density of 1.87 g/mL. If a sample has a mass of 17.5 g the volume of the sample in mL is what?
Answers
We can use the formula:
Density = Mass/Volume
Rearranging the formula gives:
Volume = Mass/Density
Substituting the given values gives:
Volume = 17.5 g / 1.87 g/mL = 9.36 mL.
Stephan’s mother cuts a twig from a rose bush and plants it in the soil. After a few days, Stephan observes a new plant growing. Which characteristic does the growth of the new plant depict?
Answers
The growth of the new plant depicts the asexual reproduction characteristic. The characteristic that describes the growth of the new plant in Stephan's mother cutting a twig from a rose bush and planting it in the soil is asexual reproduction.
Asexual reproduction is the mode of reproduction by which organisms generate offspring that are identical to the parent's without the fusion of gametes. Asexual reproduction is a type of reproduction in which the offspring is produced from a single parent.
The offspring created are clones of the parent plant, meaning they are identical to the parent.The new plant in Stephan’s mother cutting a twig from a rose bush and planting it in the soil depicts the process of asexual reproduction, which is the ability of a plant to reproduce without seeds. In asexual reproduction, plants can reproduce vegetatively by cloning themselves using their roots, bulbs, or stems.
Know more about characteristic here:
https://brainly.com/question/28790299
#SPJ8
The substitution reaction of toluene with Br2 can, in principle, lead to the formation of three isomeric bromotoluene products. In practice, however, only o- and p-bromotoluene are formed in substantial amounts. The meta isomer is not formed. Draw the structures of the three possible carbocation intermediates, and explain why ortho and para products predominate over meta products.
Answers
Answer:
See explanation and image attached
Explanation:
Aromatic hydrocarbons undergo electrophillic substitution. Usually, substituted benzene is more or less reactive to electrophillic substitution compared to unsubstituted benzene.
Substituents on the benzene ring tend to direct the incoming electrophile during electrophillic substititution. The presence of the -CH3 group on toluene directs the incoming Br electrophile to the ortho/para position.
Where the incoming electrphile E is Bromine, we can see that in the ortho/ para product, the electron pushing -CH3 stabilizes the resonance structure formed and increases electron density at the ortho/para position via resonance compared to the meta product as we can see from the image attached. Hence, the ortho and para products predominate over meta products.
Image credit: Chemistry steps

Consider the following potential energy profile for the reaction. (a) How many elementary steps are there? (b) How many intermediates are formed? (c) Which step is rate determining? (d) Is the overall reaction exothermic or endothermic?
The energy diagram contains 4 species (A, B, C, D where the energy level is A > B > C > D) and there are 3 peaks.
Answers
The overall reaction is exothermic because the energy of the products (D) is lesser than the energy of the reactants (A). The energy released during the reaction is represented by the energy difference between reactants and products, which appears as a negative value in the energy diagram.
As per the question given,
There are three peaks based on the potential energy profile, indicating that there are three basic steps in the reaction. Each peak represents a transition state where reactants convert to products.
There is an intermediate level, represented by the minimum energy between the first and second peaks. An intermediate is a short-lived compound formed during a reaction that can be further transformed into end products.
The rate-determining stage is the stage with the highest activation energy, i.e. the second peak. This is because the overall reaction rate is limited by the slower step, which requires more energy to overcome the activation barrier.
For such more questions on Reaction
https://brainly.com/question/29470602
#SPJ4
Please answer my question.

Answers
Using standard thermodynamic data at 298K, the entropy changes of the environment when 1.59 mol of P₄O₁₀ (s) is irritated at standard conditions is - 45339.5 J/K
∆H°f product is the total enthalpy of standard formation based on the product substances. ∆H°f reactants is the total enthalpy of the standard formation based on the reactants.
We know:
∆H⁰f (P₄O₁₀) = - 2984 KJ/mol
∆H⁰f (H₂O) = - 285.8 KJ/mol
∆H⁰f (4H₃PO₄) = - 1288.3 KJ/mol
The e reaction is:
P₄O₁₀ (s) + 6H₂O (l) → 4H₃PO₄ (aq)
∆H⁰rxn = ∆H⁰f products - ∆H⁰f (reactants)
∆H⁰rxn = [4∆H⁰f (P₄O₁₀)] - [1 ∆H⁰f (P₄O₁₀) + 6 ∆H⁰f (H₂O)]
∆H⁰rxn = [4(- 1288.3)] - [ 1(- 2984) + 6(- 285.8)]
∆H⁰rxn = [ - 5153.2] - [ -11936 - 1714.8]
∆H⁰rxn = [- 5153.2] - [-13650.8]
∆H⁰rxn = - 5153.2 + 13650.8
∆H⁰rxn = 8497.6 KJ
Therefore, ∆H⁰rxn for this reaction is + 8497.6 KJ.
From the above-balanced chemical equation, we can write,
For the reaction of 1 mol of P₄O₁₀,
∆H⁰rxn value = + 8497.6 KJ
Then, for the reaction of 1.59 mol of P₄O₁₀, ∆H0rxn value
= + 8497.6 KJ/1 mol x 1.59 mol
= + 13511.184 KJ
We know,
∆S⁰surroundings = - ∆H⁰rxn/ T
Where,
∆S⁰surroundings = standard entropy changes of the surroundings
∆H⁰rxn = standard enthalpy changes of the reaction
T = temperature in Kelvin
Here,
∆H0rxn = 13511.184 KJ = 13511.184 x 10³ J = 13511184 J
T = 298 K
Now,
∆S⁰surroundings = - ∆H⁰rxn/ T
∆S⁰surroundings = - 13511184J/298 K
∆S⁰surroundings = - 45339.5 J/K
Therefore, the entropy changes for the surroundings when 1.59 mol of P₄O₁₀ reacts at standard conditions is - 45339.5 J/K
Learn more about the entropy at https://brainly.com/question/6364271
#SPJ1
How many moles are in 4.6x1023 molecules of H20 expressed in the correct number
of significant figures?
Answers
We know that:
One mole of any element or compound contains 6.022*10²³ atoms/molecules
where 6.022*10²³ is also known as Avogadro's number
So, Number of moles = \(\frac{Number of atoms/molecules}{Avogadro's number}\)
Number of moles in the given sample of H₂O:
From above:
Number of moles = Number of molecules / Avogadro's number
replacing the variables
Number of moles = \(\frac{4.6 * 10^{23}}{6.022*10^{23}}\)
Number of moles = \(\frac{4.6}{6.022}\)
Number of moles = 0.7639 moles
Number of Moles in correct significant Figures:
We know that the number of significant figures of the quotient is the least number of significant figures from the numbers being divided
To find the number of moles, we divided 4.6 by 6.022
Here, 4.6 has 2 significant figures and 6.022 has 4 significant figures
Hence, the quotient(number of moles) will have 2 significant figures
Number of moles in correct significant figures = 0.76 moles
If 7 g of a gas at 2.0 ATM dissolves in 1 L of water at 25°C how much will dissolve in 2 L of water at 0.6 ATM if the temperature remains constant
Answers
The value of S2 is S₂ = 2.1 g/L.Approximately 2.1 g of the gas will dissolve in 2 L of water at 0.6 ATM, assuming the temperature remains constant.
To determine the amount of gas that will dissolve in 2 L of water at 0.6 ATM, we can use Henry's law, which states that the solubility of a gas is directly proportional to the partial pressure of the gas above the liquid.
According to Henry's law, the solubility of a gas can be represented as:S₁/P₁ = S₂/P₂,,where S₁ and S₂ are the solubilities of the gas at the respective pressures P₁ and P₂.Given that 7 g of the gas dissolves in 1 L of water at 2.0 ATM, we can consider this as our initial condition, denoted by S₁/P₁.
Now, we need to find the solubility at 0.6 ATM in 2 L of water, denoted by S₂/P₂.Since the temperature remains constant, we can assume that the solubility of the gas does not change. Therefore, we can rewrite the equation as:
S₁/P₁ = S₂/P₂,
Substituting the known values, we have:
(7 g/1 L)/(2.0 ATM) = S₂/(0.6 ATM),
Solving for S₂, we get:
S₂ = (7 g/1 L) * (0.6 ATM)/(2.0 ATM),
S₂ = 2.1 g/L.
For more such questions on temperature
https://brainly.com/question/4735135
#SPJ8
What quantity in moles of chlorine gas at 120.0 °C and 33.3 atm would occupy a vessel of 14.0 L?
Answers
A vessel of 14.0 L would hold 1.78 moles of chlorine gas at 120.0 °C and 33.3 atm.
The ideal gas law relates the pressure (P), volume (V), temperature (T), and the number of moles (n) of a gas to a constant R known as the universal gas constant. In this equation, P, V, and T are directly proportional to n, which means that as the number of moles of gas increases, so does the pressure, volume, and temperature.
Using the ideal gas law, PV = nRT, we can solve for the number of moles of chlorine gas:
n = PV/RT
First, we need to convert the temperature to Kelvin by adding 273.15:
T = 120.0 + 273.15 = 393.15 K
Next, we can plug in the values we have:
n = (33.3 atm)(14.0 L)/(0.0821 L•atm/mol•K)(393.15 K)
n = 1.78 moles
Therefore, 1.78 moles of chlorine gas at 120.0 °C and 33.3 atm would occupy a vessel of 14.0 L.
To know more about the Chlorine gas, here
https://brainly.com/question/19782746
#SPJ1
For a voltaic cell consisting of Al(s) in Al(NO3)3(aq) and Cu(s) in Cu(NO3)2(aq), what is Ecell, given [Al3 ]
Answers
Answer:
2.0 V
Explanation:
For the oxidation half cell;
Al(s) -------> Al^3+(aq) + 3e.
For reduction half cell;
Cu^2+(aq) +2e ------> Cu(s).
E°cell = E°cathode - E°anode
But;
E°cathode= 0.34 V
E°anode = -1.66 V
E°cell= 0.34 -(-1.66)
E°cell= 2.0 V
Which of the following considerations are applicable when choosing a suitable recrystallization solvent? (TRUE / FALSE) Should have a boiling point that is ~ 30-50 °C above room temperature. Does not dissolve impurities at all temperatures or completely dissolves impurities at all temperatures. Should be unreactive toward the compound of interest. Offers minimal solubility of the compound to be purified at room and lower temperatures. Its solubility-temperature relationship to the compound should give a curve with a low slope. Submit Answer Tries 0/5 In recrystallization from boiling water of benzoic acid contaminated with acetanilide, you begin with an impure sample of 5.3 grams. If the % composition of the acetanilide impurity in the sample is 3.7 %, what is the minimum amount in mL of solvent (water) required for the recrystallization? (Answer format - e.g., 33.2 mL should be entered without any units) Compound Benzoic Acid Acetanilide Solubility in water at 25C 0.34 g/100ml 0.53 g/100mL Solubility in water at 100C 5.6 g/100ml 5.5g/100 ml Your answer Submit Answer Tries 0/10 Outlined below are statements describing the general procedure followed during the purification of a solid by recrystallization. Order the process from start to finish. ، ، ، ، ، ، Remove undissolved material by gravity filtration of hot solution. Obtain the melting point of solid and calculate % recovery. Add decolorizing charcoal to the hot solution to remove the color impurity. Isolate the crystallized solid by vacuum (suction) filtration on Buchner funnel. Dissolve the impure solid in hot recrystallization solvent. Determine the approximate volume of solvent required for recrystallization.
Answers
The considerations which are applicable in a suitable recrystallization solvent are: should have a boiling point that is ~ 30-50 °C, Should be unreactive, Offers minimal solubility and solubility-temperature relationship. Option A, C, D and E will be correct.
This statement is true. Because the solvent should have a boiling point that is around 30-50 °C higher than the melting point of the compound to be recrystallized in order to achieve efficient dissolution and crystal formation.
This statement is false. Because a good recrystallization solvent should dissolve the compound of interest well at high temperatures but not at all or only slightly at lower temperatures. Ideally, the impurities should dissolve well at all temperatures, so that they can be separated from the compound of interest during the filtration step.
This statement is true. Because the solvent should not react with the compound of interest, which would affect the purity of the final product.
This statement is true. Because the solvent should have minimal solubility for the compound to be purified at room temperature and lower temperatures, but should dissolve the compound well at higher temperatures in order to achieve efficient recrystallization.
This statement is true. Because the solubility-temperature relationship for the solvent and the compound should have a low slope in order to achieve efficient recrystallization.
To know more about recrystallization here
https://brainly.com/question/14918321
#SPJ4
--The given question is incorrect, the correct question is
"Which of the following considerations are applicable when choosing a suitable recrystallization solvent? (TRUE / FALSE) A) Should have a boiling point that is ~ 30-50 °C above room temperature. B) Does not dissolve impurities at all temperatures or completely dissolves impurities at all temperatures. C) Should be unreactive toward the compound of interest. D) Offers minimal solubility of the compound to be purified at room and lower temperatures. E) Its solubility-temperature relationship to the compound should give a curve with a low slope."--
show work please energy units conversion

Answers
The answers to the given questions are; 58 Joules = 13.86 Calories, 230 Calories = 962.32 Joules, 230 Calories = 0.96 kJ, and 3.2 kJ = 0.7648 kCal.
(i) We know that, 4.184 Joules = 1 Calorie
==> 1 Joule = 1 / 4.184 Calories
Therefore, 58 Joules = [ (1 / 4.184) * 58 ] Calories
= 13.86 Calories (Approx.)
(ii) We know, 1 Calorie = 4.184 Joules
Then, 230 Calories = 4.184 * 230 Joules
= 962.32 Joules
(iii) We know, 230 Calories = 962.32 kJ(from previous question)
and, 1000Joules = 1 kJ
==> 1 Joule = (1 / 1000) kJ
Therefore, 230 Calories i.e., 962.32 Joules = [ (1 / 1000) * 962.32 ] kJ
= 0.96kJ (Approx.)
(iii) We know, 4.184 Joules = 1 Calorie
==> 1 Joule = (1 / 4.184) Calorie
==> 1000Joules i.e, 1 kJ = (1 / 4.184) * 1000 Calories
Therefore, 3.2 kJ = [ { ( 1 / 4.184 ) * 1000 } * 3.2 ] Calories
= 764.82 Calories(Approx.)
Since, 1000 Calories = 1 kCal
764.81 Calories = [(1 / 1000) * 764.82 ] kCal
i.e., 3.2 kJ = 0.7648 KCal (Approx.)
To learn more about another unit of energy:
https://brainly.com/question/26066425
how many significant figures do the following numbers have?1) 4.150 x 10-4
Answers
Significant figures correspond to the number of digits that a number contains. Zeros at the beginning and end of the number are not counted, only zeros are counted if they are in an intermediate position.
For this case, the number is written in scientific notation, the corresponding 10 of the scientific notation is not taken into account during the digit count, therefore the significant figures of this number will be:
Answer: So, in the number 4.150 x10^-4, there are 3 significant figures

How many moles of ions are contained in 1.27 L of a 1.75 M solution of Mg(NO3)2? Please answer in mol and round to the second decimal place.
Answers
There are 2.223 moles of ions contained in 1.27 L of a 1.75 M solution of Mg(NO3)2.
HOW TO CALCULATE NUMBER OF MOLES:The number of moles of an ion can be calculated by multiplying the molarity by the volume of the solution. That is:No. of moles = molarity × volume
According to this question, 1.27 L is contained in a 1.75 M solution of Mg(NO3)2. The number of moles is calculated as follows:no. of moles = 1.75M × 1.27L
no. of moles = 2.223mol
Therefore, there are 2.223 moles of ions contained in 1.27 L of a 1.75 M solution of Mg(NO3)2.
Learn more about molarity at: https://brainly.com/question/12127540
Based on the diagram below, which wave has the greatest frequency?
A. Wave B
B. They both have the same frequency.
C. Wave A
D. It is not possible to determine the frequencies of these waves.

Answers
Wave A has the greatest frequency.
What is wave ?
An energy-conducting disturbance in a medium known as a wave is one that does not involve any net particle motion. It could manifest as elasto-deformation, a shift in pressure, electric or magnetic intensity, electric potential, or temperature.
What is frequency ?
The number of full wave cycles that pass a spot in a unit of time is described as frequency. The frequency in SI is measured in Hertz (Hz).
Therefore, Wave A has the greatest frequency.
Learn more about wave from the given link.
https://brainly.com/question/26116832
#SPJ1
How does the volume and mass of reactants affect the volume of products in a chemical reaction?
Answers
According to the Le Chatlier's Principle, an equilibrium system will adapt to a change in temperature, volume, or the quantity of molecules in a reactant or product in order to reestablish balance.
Describe molecules?The smallest unit of a substance that keeps its content and properties is a molecule, which is made up of two or more atoms linked together by chemical bonds. The building blocks of chemistry are molecules. A subscript with the atom count is placed after the element symbol to identify molecules.
Simple definition of a chemical reactionWhen two or more molecules interact to create a brand-new product, a chemical reaction takes place. Reacting substances are referred to as reactants, whilst newly created substances are referred to as products.
To know about chemical reaction visit:
https://brainly.com/question/1689737
#SPJ13
How many moles of sodium hypobromite (NaBrO) should be added to 1.00 L of 0.050 M hypobromous acid
(HBrO) to form a buffer solution of pH 9.15? (Assume that no volume change occurs when the NaBrO is added)
(Ka=2.5x10)
Answers
The concentration terms are molality, normality and mole fraction. Molarity can be used to find out the ionic strength of any solution. Therefore, 0.050moles of sodium hypobromite (NaBrO) should be added to 1.00 L of 0.050 M hypobromous acid (HBrO) to form a buffer solution of pH 9.15
What is molarity?Molarity can be calculated by dividing number of moles of solute by volume of solution in litre. Molarity is affected by temperature. Its unit is mole/liter. It measure the concentration of any solute in a solution.
Mathematically,
Molarity= number of moles of solute/volume of solution in litre
Where,
moles= ?
volume= 1.00 L
Molarity=0.050 M
Substituting values in equation, we get
0.050=number of moles of solute/1.00
number of moles of solute=0.050moles
Therefore, 0.050moles of sodium hypobromite (NaBrO) should be added to 1.00 L of 0.050 M hypobromous acid (HBrO) to form a buffer solution of pH 9.15
Learn more about Molarity, here:
https://brainly.com/question/16727614
#SPJ1
How many grams of oxygen form when each quantity of reactant completely reacts?
2HgO(s)→2Hg(l)+O2(g)
Answers
When 216.59 g of HgO completely reacts, 16.00 g of O2 will be produced.
The oxygen produced
The balanced chemical equation for the reaction:
2HgO(s) → 2Hg(l) + O2(g)
states that 2 moles of HgO will produce 1 mole of O2.
We can use the molar mass of HgO and the mole ratio of HgO to O2 to calculate the mass of O2 produced from a given mass of HgO.
The molar mass of HgO is 216.59 g/mol (200.59 g/mol for Hg + 16.00 g/mol for O).
So, 1 mole of HgO has a mass of 216.59 g.
According to the balanced equation, 2 moles of HgO produce 1 mole of O2.
Therefore, 1 mole of O2 has a mass of 32.00 g.
Using stoichiometry, we can calculate the mass of O2 produced when a certain mass of HgO reacts completely.
For example, if we start with 216.59 g of HgO (1 mole), then the amount of O2 produced will be 0.5 moles (1 mole of O2 for every 2 moles of HgO), which is equivalent to 16.00 g of O2 (0.5 moles of O2 x 32.00 g/mol).
So, when 216.59 g of HgO completely reacts, 16.00 g of O2 will be produced.
Learn more on chemical reaction here https://brainly.com/question/11231920
#SPJ1
A Sample of an Organic Compound Contain
0.624 Carbon, 0.065 hydrogen, 0·028 oxygen
(a) what is the Emperical formuler of the Compound.
(b) If the relative molecular mass of the Compound Is 1940 what is the moleculer
formular of the compound (C=12₁ H=1
N = 14,0= 16)
Answers
(a) The empirical formula of the compound is \(C_{29}H_ {36}O\)
(b) The molecular formula of the compound is approximately \(C_{8}H_{118}O_{3}\)
To determine the empirical formula of the organic compound, we need to find the simplest whole-number ratio of the elements present.
(a) The given percentages of carbon, hydrogen, and oxygen can be converted into moles by dividing them by their respective atomic masses:
Carbon: 0.624 g / 12.01 g/mol = 0.052 mol
Hydrogen: 0.065 g / 1.008 g/mol = 0.064 mol
Oxygen: 0.028 g / 16.00 g/mol = 0.0018 mol
Next, we divide each of the mole values by the smallest mole value (0.0018 mol in this case) to obtain the mole ratio:
Carbon: 0.052 mol / 0.0018 mol ≈ 29
Hydrogen: 0.064 mol / 0.0018 mol ≈ 36
Oxygen: 0.0018 mol / 0.0018 mol = 1
Therefore, the empirical formula of the compound is \(C_{29}H_ {36}O\)
(b) To find the molecular formula, we need the relative molecular mass of the compound, which is given as 1940 g/mol. The empirical formula mass can be calculated by summing the atomic masses in the empirical formula:
Empirical formula mass: (29 × 12.01 g/mol) + (36 × 1.008 g/mol) + (1 × 16.00 g/mol) = 588.94 g/mol
To find the multiplier, we divide the relative molecular mass by the empirical formula mass:
Multiplier: 1940 g/mol / 588.94 g/mol ≈ 3.29
Rounding to the nearest whole number, the molecular formula of the compound is approximately 3 times the empirical formula, resulting in \(C_{8}H_{118}O_{3}\).
In summary, the empirical formula of the compound is\(C_{29}H_ {36}O\), and the molecular formula is approximately \(C_{8}H_{118}O_{3}\).
Know more about empirical formula here:
https://brainly.com/question/1439914
#SPJ8
a golf ball is hit at a distance of 300 yards in 10 seconds what is the speed of the golf ball
Answers
Answer:
30 yard per second
Explanation:
300/10 = 30