Answers
mass MgCl₂ = mol x MM MgCl₂ = 0.05 x 95.211 g/mol = 4.76 g
mass Cl in MgCl₂ :
= (2 x AM Cl)/MM MgCl₂ x mass MgCl₂
= (2 x 35.5 g/mol)/95.211 g/mol x 4.76
= 3.55 g
% mass Cl in the mixture :
= (mass Cl / mass mixture) x 100%
= 3.55 / 9.8 x 100%
= 36.22%
Related Questions
The enthalpy of vaporization for methanol is 35.2 kJ/mol. Methanol has a vapor pressure of 1 atm at 64.7 oC. Using the Clausius-Clapeyron equation, what is the vapor pressure for methanol at 55.5 oC? Give your answer in atmospheres, to the third decimal point.
Answers
Answer: 55.5 oC is 0.014 atm (3rd decimal point)
Explanation:
The Clausius-Clapeyron equation is given as:
ln(P2/P1) = -(ΔH_vap/R) * (1/T2 - 1/T1)
where:
P1 = vapor pressure at temperature T1
P2 = vapor pressure at temperature T2
ΔH_vap = enthalpy of vaporization
R = gas constant = 8.314 J/(mol*K)
Converting the enthalpy of vaporization to J/mol:
ΔH_vap = 35.2 kJ/mol = 35,200 J/mol
Converting temperatures to Kelvin:
T1 = 64.7 + 273.15 = 337.85 K
T2 = 55.5 + 273.15 = 328.65 K
Substituting the values into the equation and solving for P2:
ln(P2/1 atm) = -(35,200 J/mol / 8.314 J/(mol*K)) * (1/328.65 K - 1/337.85 K)
ln(P2/1 atm) = -4.231
P2/1 atm = e^(-4.231)
P2 = 0.014 atm
Therefore, the vapor pressure for methanol at 55.5 oC is 0.014 atm, to the third decimal point.
help me solve it pls

Answers
The term molefraction is an important method which is used to calculate the concentration of a solution. It is mainly employed to calculate the concentration of a binary solution. Here the molefraction is 1 / 5. The correct option are D, D and B.
Molefraction of any component of a solution is defined as the ratio of the number of moles of that component to the total number of moles of the solution. The sum of molefraction of solute and solvent is one.
Here the molefraction of nitrogen = Moles of nitrogen / Total number of moles
1. 'x' of 'N' = 2 / 5 + 3 + 2 = 0.2 or 1 / 5
2. Molefraction of Argon = 0.60 / 0.40 + 0.04 + 0.60 = 0.57
Partial pressure = Molefraction × Total pressure
0.57 × 6.3 = 3.59 atm
3. 20 cm³ mixture contains:
20 × 1 mole / 22400 m³ = 8.9286 × 10⁻⁴
1 mole occupies 22400 cm³
8.9286 × 10⁻⁴ × 22400 cm³ / 1 mole = 20 cm³
Thus the correct option are D, D and B.
To know more about molefraction, visit;
https://brainly.com/question/13680920
#SPJ1
A winery has a ton of wine. It cost the producers of the wine $ 30,481.92 to make the wine. If
the winery sells 5 oz. glasses of wine how much money does the winery need to charge to
double their money on the ton of wine?
Answers
A glass of wine costs about $12 at a pub. A 5-ounce glass of wine, or 147 mL, counts as one serving. This indicates that the typical bottle can accommodate five 5-ounce wine glasses. Ten wine glasses can fit within a magnum bottle. 20 glasses of wine fit inside a double-magnum bottle.
A tonne of wine weighs how much?A case of 12 wine bottles contains 9 litres, or 2.378 gallons, as a typical wine bottle is 750 millilitres in size. A tonne of grapes yields 150 gallons every tonne, which translates to 150/2.378 gallons each case, or just over 63 cases, of wine. We have a total of 756 bottles, 12 bottles each case.
How much wine does one ounce equal?Since 1 ounce equals slightly under 30 ml, a glass has a volume of 120 ml. A 750 ml bottle of wine will therefore yield slightly more than 6 glasses.
To know more about wine visit:
https://brainly.com/question/17547547
#SPJ1
determine the concentration of a solution made by dissolving 10.0 G of sodium chloride in 0.7 5L of solution
Answers
Based on the information you provided, your answer is either by mole fraction or molarity.
mole fraction:
moles a/ total moles (never greater than 1 and has no unit)
10.0g NaCl • 1 mol
——— = 0.1711156742 mol
58.44 g (molar mass of NaCl)
0.75 L solution • 1 mol
——— = 0.0334821429 mol
22.4 L (stp constant)
.1711156742 mol NaCl + 0.0334821429 mol solution = 0.2045978171 total mol
.1711156742 mol NaCl / 0.2045978171 total mol = 0.8363514168 or 0.84
0.84
molarity:
mol solute/L solution (unit- M)
10.0g NaCl • 1 mol
——— = 0.1711156742 mol
58.44 g (molar mass of NaCl)
0.1711156742 mol NaCl / 0.75 L solution = 0.2281542323 M or 0.23 M
0.23 M
hope this helps :)
The pressure of a sample of dry air is held constant 2.25 atm while the temperature is
decreased from 100°C to 7.0°C. The original volume of the sample is 43 L. Which of
the following is closest to the final volume of the sample?
A) 3.0L
B) 32L
C) 57 L
D) 610 L
Answers
V2 = V1T2/T1 = (43 L)(280.15 K)/(373.15 K)
V2 = 32 L
The correct answer choice would thus be B.
The pressure of a sample of dry air is held constant 2.25 atm while the temperature is decreased from 100°C to 7.0°C. The original volume of the sample is 43 L. 32 L is the closest to the final volume of the sample?
What is Charles's law?A law states that the volume of an ideal gas at constant pressure is directly proportional to the absolute temperature.
Charles’ law:
\(\frac{V_1}{T_1}\) = \(\frac{V_2}{T_2}\)
The volume and temperature of a fixed amount of gas at a constant pressure are directly proportional.
To use the equation, the temperature must be in Kelvin.
\(V_2\) = \(\frac{V_1}{T_1} XT_2\)
\(V_2\) = 32 L
\(V_2\)= 32 L
Hence, the correct answer is B.
Learn more about the Charles’ law here:
https://brainly.com/question/14842720
#SPJ2
What is the average mass of a single silicone atom in grams?
Answers
ANSWER QUICK!!!! GIVING BRAINIEST!!!

Answers
Answer:
B.
Explanation:
Can you give me brainliest? i need to rank up
Answer:
2nd One is correct
Which of the following affect the amount by which the freezing point of liquid is lowered by the addition of a solute? More than one answer may be correct.
A. Whether or not the compound is ionic.
B. How soluble the solute is in the solvent.
C. The volume of the solvent.
D. The value of the freezing point for the pure solvent.
E. The identity of the chemical species being dissolved.
Answers
The options that affect the amount that the freezing point is lowered by the addition of the solute include :
A. Whether or not the compound is ionic.B. How soluble the solute is in the solvent.How can solutes affect the freezing point of liquids ?The increasing of a solvent's boiling point as a result of the addition of a solute is known as boiling point elevation. Similar to freezing point depression, adding a solute lowers the freezing point of a solvent. In actuality, a solvent's freezing point drops as its boiling point rises.
Any solvent's freezing point will be lowered by the presence of a solute; this action is known as freezing-point depression. The fact that the solute is present in the liquid solution but not in the pure solid solvent is crucial to understanding this phenomenon.
This includes the compound being ionic or the solubility of the solvent.
Find out more on freezing point at https://brainly.com/question/40140
#SPJ1
Data for CH3COOH(l) + C2H5OH(l) CH3COOC2H5(l) + H2O(l) balance were obtained at 100. The initial concentrations of the reagents are indicated in columns 1 and 2 of the table below and the CH3COOC2H5 concentrations in equilibrium are given in column 3. Calculate H2O and determine the value of KC

Answers
Answer:
Kc = 3.94
Explanation:
CH₃COOH(g) + C₂H₅OH(g) → CH₃COOC₂H₅(g) + H₂O(g)
Liquids aren't included in the equilibrium constant, but at 100°C, all four compounds are a gas. So the equilibrium equation is:
Kc = [CH₃COOC₂H₅] [H₂O] / ([CH₃COOH] [C₂H₅OH])
Set up an ICE table for each row and calculate the value of Kc. Then average the results. (See picture.)
Kc ≈ (3.919 + 4.012 + 3.902) / 3
Kc ≈ 3.94

3) The cathode of a voltaic cell has a strip of Fe(s) immersed in an unknown concentration of Fe3+ (aq) and the anode is a standard Zn half cell. The potential of the cell is 0.65V. If the standard cell potential for a Zn/Fe cell is 0.72V, what is the molar concentration of Fe3+?
Answers
The molar concentration of Fe3+ in the solution is approximately 230.82 M.
To calculate the molar concentration of Fe3+, we can use the Nernst equation, which relates the cell potential to the concentrations of the species involved in the cell reaction.
The Nernst equation is given as:
Ecell = E°cell - (0.0592/n) * log(Q)
Where:
Ecell is the measured cell potential (0.65V in this case),
E°cell is the standard cell potential (0.72V in this case),
n is the number of electrons transferred in the cell reaction (in this case, it is 2, as Fe3+ gains 2 electrons to form Fe),
Q is the reaction quotient.
In this case, the reaction at the cathode is:
Fe3+(aq) + 2e- -> Fe(s)
And the reaction quotient Q can be expressed as the molar concentration of Fe3+:
Q = [Fe3+]
Substituting the given values into the Nernst equation, we have:
0.65V = 0.72V - (0.0592/2) * log([Fe3+])
Simplifying the equation:
0.65V = 0.72V - 0.0296 * log([Fe3+])
0.0296 * log([Fe3+]) = 0.07V
log([Fe3+]) = 0.07V / 0.0296
log([Fe3+]) ≈ 2.3659
Taking the antilog (inverse logarithm) of both sides:
[Fe3+] ≈ 10^(2.3659)
[Fe3+] ≈ 230.82
for more such questions on solution
https://brainly.com/question/25326161
#SPJ11
A solution has 0.0048 M of H+. What is the pH? Is
the solution acidic or basic?
Answers
Answer:
Explanation:
The pH of a solution is the negative logarithm (base 10) of its hydrogen ion concentration (H+):
pH = -log[H+]
In this case, the hydrogen ion concentration is 0.0048 M, so we can calculate the pH as:
pH = -log(0.0048) = 2.32
Therefore, the pH of the solution is 2.32.
Since the pH is less than 7, the solution is acidic.
the pka is the ph at which a weak acid’s protons are 50% titrated. t or f
Answers
The statement 'the pKa is the pH at which a weak acid’s protons are 50% titrated' is true as weak acids do not have the ability to get completely ionized.
The statement is true because weak acids do not completely ionize in solution, so at a certain pH, half of the acid’s protons will be titrated.
This is known as the acid’s dissociation constant, which is a measure of the acid’s ionization in solution. The pKa is the pH at which the acid’s dissociation constant is equal to one.
The lower the pKa, the more acidic the solution will be. A weak acid with a pKa of 2 will be more acidic than one with a pKa of 5.
Therefore, the statement 'the pKa is the pH at which a weak acid’s protons are 50% titrated' is true.
To learn more about pH , click here
https://brainly.com/question/15289741
SPJ4
A sample of sodium chloride was found to contain 60.66 g of sodium and 39.34 g of chloride. How many
grams of each element would be expected in a 200.0 g sample of sodium chloride?
Answers
Answer:
78.68g Na
121.32 Cl
Explanation:
This one is more easy than the wording makes it seem. Since there are 60.66g of sodium (Na) and 39.34g of Chlorine (Cl) it adds up to 100g, so in this case you can just double each amount, and get what each would be in a 200g sample.
Speed can be though of as ____ the at which an object covers a _____.
A) speed,kilometer
B) Rate,distance
C) Forces, distance
D) Distance, rate
Pls help me with this question like now thank
Answers
Answer:
Speed can be thought of as the rate at which an object coverse distance
B) Reate distance
a polluted lake is 0.300 μg (micrograms) per liter of water, what is the total mass of mercury in the lake, in kilograms, if the lake has a surface area of 15.0 square miles and an average depth of 27.0 feet?
Answers
Answer:
95.9 kg
Explanation:
First we convert 15.0 mi² to m²:
15.0 mi² * (\(\frac{1609.34 m}{1mi}\))² = 3.88x10⁷ m²Then we convert 27.0 ft to m:
27.0 ft * \(\frac{0.3048m}{1ft}\) = 8.23 mNow we calculate the total volume of the lake:
3.88x10⁷ m² * 8.23 m = 3.20x10⁸ m³Converting 3.20x10⁸ m³ to L:
3.20x10⁸ m³ * \(\frac{1000L}{1m^3}\) = 3.20x10¹¹ LNow we calculate the total mass of mercury in the lake, using the given concentration:
0.300 μg / L * 3.20x10¹¹ L = 9.59x10¹⁰ μgFinally we convert μg to kg:
9.59x10¹⁰ μg * \(\frac{1kg}{1x10^9ug}\) = 95.9 kgPropane is used as a fuel for camp stoves. It undergoes combustion to form carbon dioxide and water.
C3H3 +502 – 3 CO2 + 4H20
Determine the number of molecules of propane needed to produce 10.01 liters of carbon dioxide
Answers
Answer:
\(8.97x10^{22}molecules C_3H_8\)
Explanation:
Hello there!
In this case, according to the given chemical reaction and the fact that 22.4 L of a gas are occupied by 1 mol at standard pressure and temperature conditions, it will be possible for us to calculate the number of molecules of propane, by using the Avogadro's number, the 1:3 mole ratio with carbon dioxide and the aforementioned volume-mole ratio to obtain:
\(10.01LCO_2*\frac{1molCO_2}{22.4LCO_2}*\frac{1molC_3H_8 }{3molCO_2} *\frac{6.022x10^{23}molec\ C_3H_8}{1molC_3H_8} \\\\=8.97x10^{22}molecules C_3H_8\)
Regards!
Does the model represent a chemical reaction? (Image)
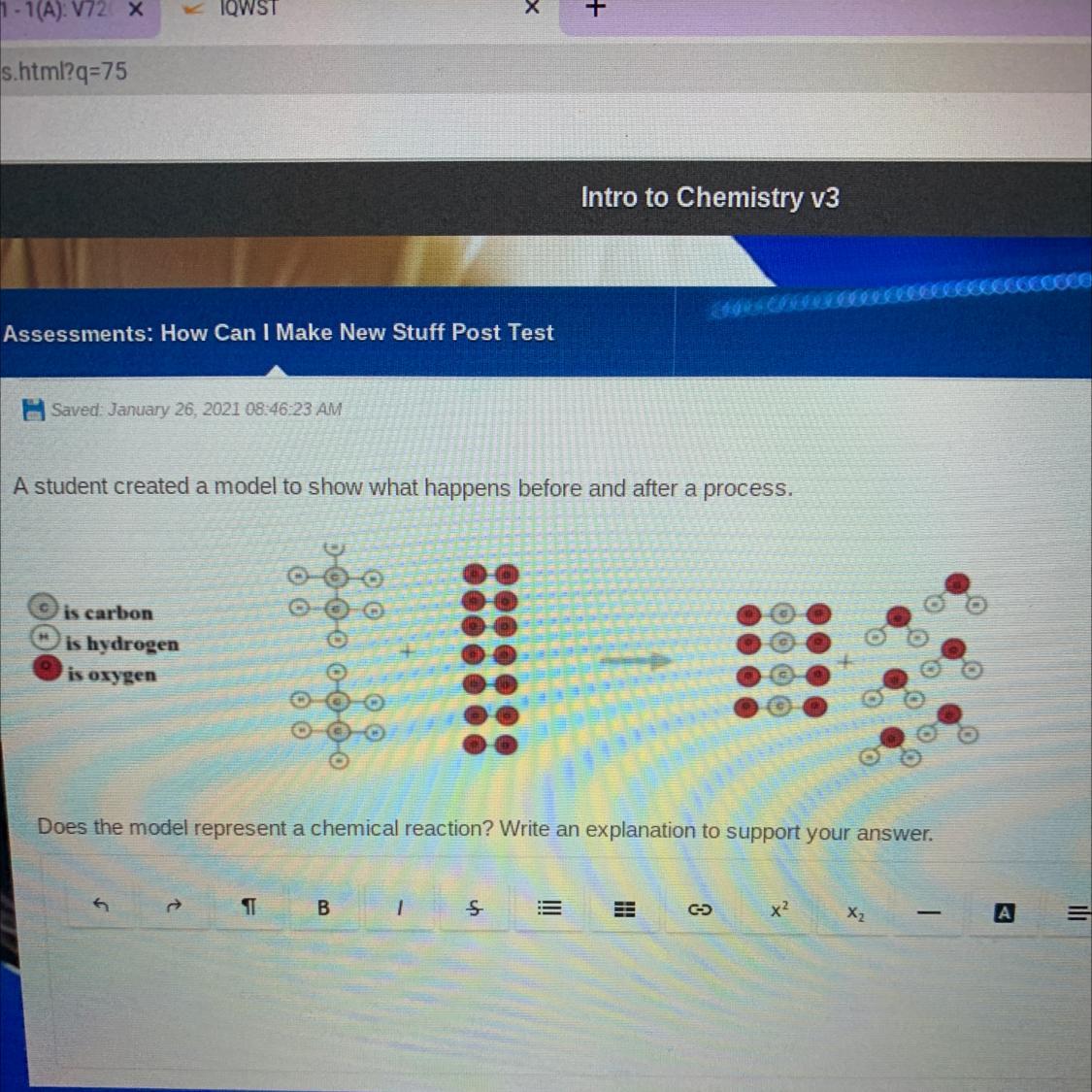
Answers
Answer:
yes it is a chemical reaction
Explanation:
because the substances combined and made something new
Suppose that the microwave radiation has a wavelength of 12.4 cm. How many photons are required to heat 205 mL of coffee from 25.0 ∘C to 62.0 ∘C ? Assume that the coffee has the same density, 0.997 g/mL, and specific heat capacity, 4.184 J/(g⋅K), as water over this temperature range.
Answers
Answer:
To calculate the number of photons required to heat the coffee, we can follow these steps:
Calculate the mass of the coffee using its volume and density:
mass = volume x density = 205 mL x 0.997 g/mL = 204.185 g
Calculate the amount of heat required to raise the temperature of the coffee using its mass, specific heat capacity, and temperature change:
q = m x c x ΔT = 204.185 g x 4.184 J/(g⋅K) x (62.0 - 25.0) °C = 32289.6 J
Calculate the energy of each photon using the formula E = hc/λ, where h is Planck's constant, c is the speed of light, and λ is the wavelength of the microwave radiation:
E = (6.626 x 10^-34 J⋅s) x (3.00 x 10^8 m/s) / (0.124 m) = 5.067 x 10^-23 J
Calculate the number of photons required to deliver the amount of energy needed to heat the coffee:
number of photons = q / E = 32289.6 J / 5.067 x 10^-23 J = 6.368 x 10^25 photons
Therefore, approximately 6.368 x 10^25 photons are required to heat 205 mL of coffee from 25.0 ∘C to 62.0 ∘C using microwave radiation with a wavelength of 12.4 cm.
A sample of 0.35 L of argon gas (at a temperature of 13 oC and a pressure of 568 torr) is heated to 156 oC and a new pressure of 897 torr. Calculate the new volume of the gas.
Answers
Answer:
The new volume of the gas is 0.332 liters.
Explanation:
Let suppose that argon behaves ideally, the equation of state of the ideal gas is:
\(P\cdot V = n \cdot R_{u}\cdot T\)
Where:
\(P\) - Pressure, measured in torr.
\(V\) - Volume, measured in liters.
\(n\) - Molar quantity, measured in moles.
\(T\) - Temperature, measured in kelvins.
\(R_{u}\) - Ideal gas constant, measured in \(\frac{torr\cdot L}{mol \cdot K}\).
Since gas sample is a closed system experimenting a heating process, that is, molar quantity remains constant, the following relationship is derived from the equation of state described above:
\(\frac{P_{o}\cdot V_{o}}{T_{o}} = \frac{P_{f}\cdot V_{f}}{T_{f}}\)
Where:
\(P_{o}\), \(P_{f}\) - Initial and final pressures, measured in torr.
\(V_{o}\), \(V_{f}\) - Initial and final volumes, measured in liters.
\(T_{o}\), \(T_{f}\) - Initial and final temperatures, measured in kelvins.
Now, the final volume of the gas is found:
\(V_{f} = \left(\frac{T_{f}}{T_{o}}\right)\cdot \left(\frac{P_{o}}{P_{f}} \right)\cdot V_{o}\)
If \(T_{o} = 286.15\,K\), \(T_{f} = 429.15\,K\), \(P_{o} = 568\,torr\), \(P_{f} = 897\,torr\) and \(V_{o} = 0.35\,L\), the new volume of the gas is:
\(V_{f} = \left(\frac{429.15\,K}{286.15\,K} \right)\cdot \left(\frac{568\,torr}{897\,torr} \right)\cdot 0.35\,L\)
\(V_{f} = 0.332\,L\)
The new volume of the gas is 0.332 liters.
A state of matter where the particles that make up a substance start to break apart
Answers
Answer:
Liquid
Explanation:
how much heat will be absorbed by a 52.7 g piece of aluminum as it changes temperature from 23.0 c to 67.0c
Answers
The amount of heat absorbed by 52.7 g of aluminum as it changes temperature from 23.0 c to 67.0c is solved to be 2044.48 J.
What is specific heat capacity?For thermodynamic investigations, scientists needed a measurement that was independent of the quantity or size of the material being considered, therefore they created the concept of specific heat capacity.
Being unaffected by the quantity or size of the matter makes it an intense quality. A material's or matter's specific heat capacity is the amount of heat energy needed to raise the temperature of a unit mass of that substance by one degree Celsius.
To calculate the heat absorbed by a piece of aluminum as its temperature changes, use the formula:
Q = mcΔT
where:
Q is heat
m is mass (52.7 g)
c is specific heat capacity of aluminum (0.90 J/g°C)
ΔT is change in temperature (67.0°C - 23.0°C = 44.0°C)
Plugging in the values, we get:
Q = 52.7 g * 0.90 J/g°C * 44.0°C = 2044.48 J.
So, the 52.7 g piece of aluminum absorbs 2044.48 J of heat as its temperature changes from 23.0°C to 67.0°C.
Learn more about specific heat capacity at:
https://brainly.com/question/14646878
#SPJ1
How many protons, neutrons, and electrons are in Phosphorous-33?
Answers
Answer:
It has 17 protons, 17 electrons, and 18 neutrons/ 35 protons, 35 electrons, and 17 neutrons. 21. Phosphorus-33 (atomic number 15) contains 18 neutrons/ 18 protons
what is the volume of the rock? if the water rose from 50L to 70mL
Answers
Which development started a large movement of settlers to California?(1 point)
Responses
manifest destiny
manifest destiny
gold rush
gold rush
cattle drive
cattle drive
Transcontinental Railroad
Answers
The Transcontinental Railroad was the main development that started a large movement of settlers to California. It was the first railway line to span the entire continent, connecting the east and west coasts of the United States.
The railroad greatly facilitated migration and allowed for widespread settlement of the West. It improved communication between the east and west coasts, sped up transportation, and made it possible for a more productive interchange of goods.
Also, the railroad made it possible for prospectors to reach California in search of the California Gold Rush. Also, the railroad made it possible for cattle ranchers to transport their herds of cattle to the California stockyards.
As a result, there was more beef available, which aided in the state's agriculture sector's expansion. Also, the railroad offered an effective way to ship mining and agricultural goods to the east coast.
Hence, the Transcontinental Railroad served as the main driving force behind the massive migration of settlers to California.
To learn more about California visit:
https://brainly.com/question/30374131
#SPJ1
PLEASE HELP!!! WILL MARK BRIANLIST DO NOT GUESS I HAVE ATTEMPTED THREE TIMES

Answers
6. A. If 4.50 mols of ethane, C2H6, undergoes combustion according to the unbalanced equation
C2H6 + O2 ----------> CO2 + H2O
how many moles of oxygen are required?
B. How many moles of each product are formed?
Answers
Based on the mole ratio of the balanced equation of the reaction,
4.5 moles of ethane will require 15.75 moles of oxygenMoles of carbon (iv) oxide, CO₂, produced is 9 molesMoles of water, H₂O, produced is 13.5 molesWhat is the mole ratio of the reaction?The mole ratio of the reaction is obtained from the balanced equation of the reaction as follows:
Balanced equation of reaction: 2 C₂H₆ + 7 O₂ ------> 4 CO₂ + 6 H₂O
From the balanced equation of the reaction, the mole ratio of the reaction shows that 2 moles of ethane, C₂H₆, undergo combustion with 7 moles of oxygen, O₂, to produce 4 moles of carbon (iv) oxide, CO₂, and 6 moles of water, H₂O.
Hence;
4.5 moles of ethane will require 4.5 * 7/2 moles of oxygen
moles of oxygen required = 15.75 moles of oxygen
Moles of carbon (iv) oxide, CO₂, produced = 4.5 * 4/2
Moles of carbon (iv) oxide, CO₂, produced = 9 moles
Moles of water, H₂O, produced = 4.5 * 6/2
Moles of water, H₂O, produced = 13.5 moles
Learn more about mole ratio at: https://brainly.com/question/26023
#SPJ1
Please Need help asap
For Al, its atomic number is 13 and its mass number is 27. How many neutrons does it have?
А. 13
B. 14
C. 26
D. 27
E. 40
Answers
Answer:
A
Explanation:
The number of protons and neutrons of an element is the same. the electrons are the only thing that can differ. The atomic number equal the protons and neutrons.
When a statement is represented with molecules and symbols, the representation is called what?
Answers
Define Chemical Formula
Using atom symbols and numerical subscripts, a chemical equation is a notation used by chemists to show the number and kind of atoms present in a molecule. A written chemical equation is a straightforward illustration of an actual, three-dimensional molecule. The precise atoms that make up a material are described in its chemical formula. The equation, the chemical formula, and the methodology was introduced are the three fundamental types of chemical formulas.
Each of these chemical formulas offers a little bit of a different insight into a substance's composition, hints at its three-dimensional shape, and information about how it will interact with other molecules, atoms, and ions. The letters in a chemical formula stand in for each atom's atomic symbol. Each atom's number is represented by the subscript (lower), while its charge is represented by the superscript (higher). A coefficient placed before a chemical formula denotes the number of molecular units in the compound. The way that each type of chemical formula is read varies slightly.
to know more about molecule, visit to:-
https://brainly.com/question/19922822
#SPJ1
How many moles of Carbon do you have if you contain 72 grams of Carbon?
Answers
Answer:
6 moles of Carbon.
Explanation:
One mole of Carbon is equal to 12 grams of Carbon, so you would divide 72 by 12 and the answer would be 6.
Theoretical yield
2.05 g salicylic acid x (180g aspirin/1 mol) x (1 mol/138 g salicylic acid)
Answers
The question is incomplete; part of the data required in the question are shown:
Theoretical Yield: 2.05 g salicylic acid x (180g aspirin/1 mol) x (1 mol/138 g salicylic acid) 2. Mass of filter paper 2.56 g 3. Mass of filter paper and aspirin 5.42 g 4. Mass of aspirin (3-2) g. Percent Yield [(4)/(1)] x 100
Answer:
107%
Explanation:
We can calculate the theoretical yield as shown;
2.05g salicylic acid × 180g aspirin/1mol × 1 mol/138g of salicylic acid
Theoretical yield= 2.67 g of aspirin
Actual yield of aspirin is obtained from the experimental data;
Mass aspirin + filter paper= 5.42 g
Mass of filter paper= 2.56 g
Mass of aspirin= 5.42 g -2.56 g = 2.86 g
Hence actual yield of aspirin = 2.86 g
Percentage yield = actual yield/theoretical yield × 100
Percentage yield = 2.86/2.67 ×100 = 107%