A sample of gas is placed in a rigid container. If the original conditions were 320 torr and 400 K, what will be the pressure in the container at 200 K?
a. 160 torr
b. 640 torr
c. 250 torr
d. 760 torr
Answers
P₁V₁/T₁ = P₂V₂/T₂
where P₁ and T₁ are the initial pressure and temperature, P₂ and T₂ are the final pressure and temperature, and V₁ and V₂ are the initial and final volumes (assuming constant volume in this case since the container is rigid).
Let's plug in the values given:
P₁ = 320 torr
T₁ = 400 K
T₂ = 200 K
Since the volume is constant, V₁ = V₂, so we don't need to include it in the equation.
Now, we can solve for P₂:
P₁/T₁ = P₂/T₂
P₂ = (P₁ * T₂) / T₁
= (320 torr * 200 K) / 400 K
= 160 torr
Therefore, the pressure in the container at 200 K would be 160 torr (option a).
Related Questions
Explain why malting point and poiling of alkane increae with increaing number of carbon atom
Answers
A lot of energy is required to overcome these forces and melt or vaporize the alkane
The melting point and boiling point of alkanes (a type of hydrocarbon with only single bonds between the carbon atoms) generally increase as the number of carbon atoms in the molecule increases. This is because the larger the molecule, the more Van der Waals forces (also known as London dispersion forces) that are present. These forces are a type of weak attractive force that can exist between any two molecules and are caused by the fluctuating dipoles (temporary separations of positive and negative charge) that occur within a molecule. The more carbon atoms a molecule has, the more fluctuating dipoles it has, and the stronger the Van der Waals forces are. As a result, more energy is required to overcome these forces and melt or vaporize the substance.
In addition to the size of the molecule, the type of bond present in the molecule can also affect the melting point and boiling point. Single bonds, like those found in alkanes, are weaker than double bonds or triple bonds. As a result, molecules with double or triple bonds have higher melting points and boiling points than molecules with the same number of carbon atoms but only single bonds.
Learn more about Alkanes here: brainly.com/question/3648919
#SPJ4
14. Which of the following statement is correct?
(a) Materials existing as liquids at room temperature have their melting and boiling points lower than
that of room temperature.
(b) The phenomenon involving the transition of a substance from solid to liquid state is called
sublimation
(c) To convert a temperature on the Celsius scale to Kelvin scale, subtract 273 from the given
temperature
(d) The density of ice is less than that of water.
Answers
Answer:
(d)
Explanation:
The correct statement is the density of ice is less than that of water. Therefore, option D is correct.
Why is the density of ice less than that of water?Water has a higher density than ice because its molecules are more tightly packed. Don't be fooled by the fact that ice is a solid. Water expands as it freezes. As a result, ice has a higher volume than water.
Because the orientation of hydrogen bonds causes molecules to push farther apart, ice is less dense than water.
Other liquids' solid states are denser because the molecules pack together tightly as the kinetic energy (temperature) decreases. Although the hydrogen bonds in water ice are strong, their orientation causes molecules to push apart, lowering density.
Thus, option D is correct.
To learn more about the density of ice, follow the link;
https://brainly.com/question/11696500
#SPJ6
Particles of a liquid
A. Are free to move in a container but remain in close contact with one another
B. Are tightly packed together and stay in a fixed position
C. Have no viscosity
D. Decrease in volume with increasing temperature
Answers
Answer:
A
Explanation:
B. That's a solid
C. Liquid is the only thing that can have viscosity
D. Not necessarily the case
How did John Smith put the rule of law into action in Jamestown?
Responses
He decided to have the laws apply only to people who did not own land.
He decided to have the laws apply only to people who did not own land.
He declared himself to be above the law as the governor of Jamestown.
He declared himself to be above the law as the governor of Jamestown.
He wrote the Jamestown Constitution, which guaranteed equal protection under the law.
He wrote the Jamestown Constitution, which guaranteed equal protection under the law.
He said that everyone, including himself, needed to work if they wanted to eat.
Answers
The way that John Smith put the rule of law into action in Jamestown is by option D: He said that everyone, including himself, needed to work if they wanted to eat.
What is John Smith rule about?He imposed the rule "He who will not work must not eat" in order to create stricter discipline among the settlers. The colony advanced under Smith's leadership: the settlers dug the first well, sowed crops, and started rebuilding the fort that had burned down the winter before.
Therefore, He was known for implementing strict discipline in the colony, including the "law of the harvest" which stated that everyone, including himself, needed to work if they wanted to eat. However, there is no evidence that he wrote a constitution or that he declared himself to be above the law as governor.
Learn more about John Smith from
https://brainly.com/question/17837412
#SPJ1
PLEASE HELP this is due today the picture is below
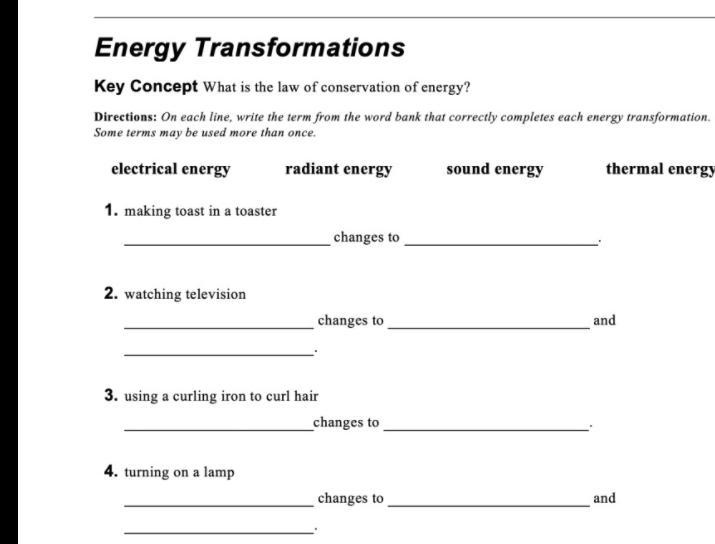
Answers
Answer:
Explanation:
Ok so the first one would be Electrical energy and Radiated energy
Then the 2 question it would be not electrical but the other 3 for the 3 question it would be it would be Electrical and the radiated energy
And for the last one it would be the last 3
A 16.1 mL sample of an HCl solution is found to contain 3.0 x 10-3 mol HCl. What is the molarity of this solution
Answers
Taking into account the definition of molarity, the molarity of the solution is 0.186\(\frac{moles}{liter}\).
Definition of molarityMolar concentration or molarity is a measure of the concentration of a solute in a solution and indicates the number of moles of solute that are dissolved in a given volume.
The molarity of a solution is calculated by dividing the moles of solute by the volume of the solution:
\(Molarity=\frac{number of moles}{volume}\)
Molarity is expressed in units \(\frac{moles}{liter}\).
Molarity of the solution in this caseIn this case, you know:
number of moles= 3×10⁻³ molesvolume= 16.1 mL= 0.0161 LReplacing in the definition of molarity:
\(Molarity=\frac{3x10^{-3} moles}{0.0161 L}\)
Solving:
molarity= 0.186\(\frac{moles}{liter}\)
Finally, the molarity of the solution is 0.186\(\frac{moles}{liter}\).
Learn more about molarity:
brainly.com/question/9324116
brainly.com/question/10608366
brainly.com/question/7429224
the ______ heat capacity of a substance is the amount of heat needed to change the temperature of 1 mol of that substance by ___ K
molar ; 1
Answers
The molar heat capacity of a substance is the amount of heat needed to change the temperature of 1 mol of that substance by 1 degree Celsius (or Kelvin).
Molar heat capacity is termed as the amount of heat energy will be required to raise the temperature of one mole of a substance by one degree Celsius (or one Kelvin). It is typically denoted by the symbol Cₘ and has units of J/mol·K.
The molar heat capacity will be related to specific heat capacity, which is the amount of heat energy will be required to raise the temperature of one gram of the substance by one degree Celsius (or one Kelvin). The specific heat capacity can be converted to the molar heat capacity by dividing it by the molar mass of the substance.
To know more about Molar heat capacity here
https://brainly.com/question/1792547
#SPJ4
Synthesis of 4-Nitroveratrole Digital Lab Report Chem 2550 Mechanism. Propose a mechanism for the following reaction and label the rate-determining step. Make sure to include (1) the formation of the electrophile and (2) all significant resonance contributors of the sigma complex intermediate. (10 points) NOZ HNO3 (1 eq.) H2SO4 0°C
Answers
The mechanism for the synthesis of 4-nitroveratrole involves the formation of an electrophile followed by the formation of a sigma complex intermediate, which is the rate-determining step.
Mechanism:
1. Formation of Electrophile: The nitration of 4-nitroveratrole starts with the formation of an electrophile by protonation of nitric acid (HNO3) with sulfuric acid (H2SO4). The protonated nitric acid (HNO3 + H2SO4) acts as an electrophile.
2. Sigma Complex Formation: The electrophile then reacts with the aromatic ring of 4-nitroveratrole to form a sigma complex intermediate. The sigma complex intermediate has significant resonance contributors, including the positively charged nitrogen atom and the delocalized electrons of the aromatic ring.
Rate-Determining Step: The formation of the sigma complex intermediate is the rate-determining step. This step is rate-limiting because it involves a reaction between an electron-rich aromatic ring and an electrophile, which is a slow reaction due to the stability of the aromatic ring.
To know more about electrophile please refer: https://brainly.com/question/30026567
#SPJ4
How much water would you need to add to 950 mL of a 3.500 M sodium chloride solution to make a 2.500 M solution?
Answers
You would need to add 250 mL of water.
CH4 (g) + O2 (g) → H2O (l) + CO2 (g)
This is an example of:
A. Synthesis
B. Combustion
C. Double replacement
D. Decomposition

Answers
Answer:
B. Combustion.
Explanation:
Looking at the given equation, we can see that methane (CH4) reacts with oxygen (O2) and releases water (H2O) and carbon dioxide (CO2). This matches the definition of a combustion reaction. Therefore, the answer is B. Combustion.
Using the thermodynamic information in the ALEKS Data tab, calculate the boiling point of bromine (Bry). Round your answer to the nearest degree___
Answers
The boiling point of bromine (Br₂) is approximately 59°C. This value is derived from the thermodynamic information provided in the ALEKS Data tab.
To calculate the boiling point of bromine, we can use the thermodynamic data provided in the ALEKS Data tab. The boiling point of a substance is the temperature at which its vapor pressure equals the atmospheric pressure. Bromine's vapor pressure can be determined using the Clausius-Clapeyron equation, which relates the vapor pressure of a substance to its temperature and enthalpy of vaporization. Using the data tab, we can find the enthalpy of vaporization for bromine (ΔHvap = 29.54 kJ/mol) and use it in the equation along with the known vapor pressure and atmospheric pressure to calculate the boiling point. After solving for the temperature, rounding the answer to the nearest degree gives us the boiling point of bromine as 59°C.
know more about boiling point of bromine, here:
https://brainly.com/question/10643687
#SPJ11
what is the physical state of element chromium
Answers
Answer:
Solid metal.
Explanation:
At room temperature, Chromium (Cr), a transition metal, is a solid.
how much heat is needed to convert 906 of ice at to steam at ? be sure your answer has the correct number of significant digits.
Answers
Amount of heat energy that is required to heat 906 g of ice to steam is 530952 Joules.
Explanation:
Given:
Amount of transferred energy = Q =?
Mass of ice (water) = m = 906 g
Initial temperature of ice = T(i) = -22° C
Final temperature of steam = T(f) = 118°C
Formula for Heat capacity is given by
Q = m×c×Δt ........................................(1)
where:
Q = Heat capacity of the substance (in J)
m=mass of the substance being heated in grams(g)
c = the specific heat of the substance in J/(g.°C)
Δt = Change in temperature (in °C)
Δt = (Final temperature - Initial temperature) = T(f) - T(i)
= 118 - (-22) = 140°C
Specific heat of water is c = 4.186 J /g. °C
Substituting these in equation (1), we get
Q = m×c×Δt
= 906 × 4.186 × 140
= 530952.24 J
The heat energy required is equal to 530952.24 Joules ≅ 530952 Joules.
Learn more about heat energy from the link given below.
https://brainly.com/question/18757266
#SPJ4
If there is something floating in a liquid, what is the best way to separate the two chemicals?
-distillation
-evaporation
-filtration
-sorting
Answers
Answer:
Filtration
explanation:
Filtration is a separation method used to separate out pure substances in mixtures comprised of particles—some of which are large enough in size to be captured with a porous material. Particle size can vary considerably, given the type of mixture. For instance, stream water is a mixture that contains naturally occurring biological organisms like bacteria, viruses, and protozoa. Some water filters can filter out bacteria, the length of which are on the order of 1 micron. Other mixtures, like soil, have relatively large particle sizes, which can be filtered through something like a coffee filter.
is aluminium more reactive than copper?
Answers
Answer:
Yes aluminum is reactive than copper
Answer: Aluminium is more reactive than Copper.
More in details:
Aluminium is located much above in reactivity series of metal than Copper.So, Aluminium is more reactive than Copper.
2 chemical properties or chlorine as well as what it reacts with
Answers
Answer:
Chlorine reacts with most metals and forms metal chlorides, with most of these compounds being soluble in water. Examples of insoluble compounds include AgCl and PbCl2. Gaseous or liquid chlorine usually does not have an effect on metals such as iron, copper, platinum, silver, and steel at temperatures below 230°F.
Explanation:
Given: sulfur, 3.04 g, 1.47 cm³
Wanted: density of sulfur in g/cm³?
Answers
Answer:
2.068
Explanation:
D = M/V
3.04 /1.47
= 2.068
below given are some example of solution identify solute and solvent from them also mention whether they are solid, liquid and gas . Air,milk,water, pepsi,humid air,sea water, polluted air
Answers
Answer:
Solutes examples are
Air,(gas) humid air (gas), polluted air(gas)
Examples of Solvents are Milk(liquid), water(liquid).,
Pepsi(liquid).
Sea water(liquid)
Explanation:
Solutes are substances that are dissolved and it is present in minute fraction in a solution.
Solvents is the medium that dissolve substances or solutes and it is present in large amount in a solution.
Solutes and solvents for solution.
Solution is formed when substances dissolves in another.
Air: The solvent is nitrogen because it is present in large quantities and the solutes is oxygen because it is present in small quantity.
Pepsi :sugar, caffeine and carbon dioxide as solutes and the solvent is water.
Milk: lactose, some minerals are solutes and Water is the solvent.
Humid air: water is the solutes and gases is the solvent.
Sea water: salt is the solute and water is the solvent.
Polluted air: Air is the solvent and dust particles is the solutes.
A certain first order reaction, A ----> B, when performed at 25oC, is 46% complete after 68 min. What is the rate constant of this reaction?
Answers
The rate constant for the reaction at is 0.009 min-1
The rate constant of the first order reaction A ----> B performed at 25oC can be calculated using the following equation: Rate constant (k) = ln(2)/ t1/2
where t1/2 is the time taken for the reaction to be 50% complete.
In this case, the reaction is 46% complete after 68 minutes. To calculate the rate constant, we need to calculate the time taken for the reaction to be 50% complete. This can be calculated as follows:
t1/2 = 68 x (50/46)
t1/2 = 75.4 minutes
Therefore, the rate constant for the reaction at 25oC can be calculated as follows:
k = ln(2)/75.4 = 0.009 min-1
To know more about rate constant click on below link:
https://brainly.com/question/14977272#
#SPJ11
Sam loves his mom’s cookies. One day, after eating ten cookies, he stopped eating. His mom offered him some more, but he refused to eat them. Which statement best explains Sam’s behavior?
Answers
After eating ten cookies, Sam refused to eat more cookies because he reached the satiation point, the point where the body signals to the brain that it has had enough food for that particular meal. This process helps regulate the amount of food we eat, preventing us from overeating, which can lead to health problems.
Satiation is a significant component of hunger and appetite control. It is controlled by the hypothalamus, which receives signals from the digestive system regarding the availability of nutrients in the body. When we eat, the stomach expands, sending signals to the hypothalamus, which results in the production of satiety hormones like leptin and insulin, which are responsible for regulating hunger and fullness levels in the body.Therefore, when Sam refused to eat more cookies, it was because he had reached his satiation point, and his body signaled to his brain that he had consumed enough cookies. As a result, his hunger and appetite were satisfied, and he did not want to eat more cookies.For more such questions on overeating, click on:
https://brainly.com/question/13476492
#SPJ8
How
many grams of KCI are needed to make 600mL of a 15% solution
Answers
To determine the number of grams of KCI needed to make a 15% solution in 600 mL, we can use the equation:
grams of solute = (volume of solution in mL) x (concentration of solution in decimal form)
First, we need to convert the given volume of the solution from mL to liters by dividing it by 1000:
600 mL ÷ 1000 = 0.6 L
Now we can calculate the grams of KCI required:
grams of KCI = 0.6 L x 0.15
grams of KCI = 0.09 L
Therefore, to make a 15% solution in 600 mL, you would need 0.09 grams of KCI.
The formula used above is based on the definition of concentration, which states that the concentration of a solution is equal to the amount of solute divided by the volume of the solution. By rearranging the equation, we can solve for the amount of solute (grams of KCI) needed for a given concentration and volume of the solution.
To know more about number of grams of KCI click this link-
https://brainly.com/question/24081006
#SPJ11
Familiarity with common functional groups is key to understanding the physical and chemical properties of the molecules that contain them. Identify the carboxylic acid functional group. H H Н Selected Coordinates Clear QUESTION 3 Copy of Familiarity with common functional groups is key to understanding the physical and chemical properties of the molecules that contain them. Identify the amine functional group N. H Selected Coordinates Clear QUESTION 4 Familiarity with common functional groups is key to understanding the physical and chemical properties of the molecules that contain them. Identify the ester functional group H Н Selected Coordinates Clear
Answers
Carboxylic acid functional group: -COOH
Amine functional group: -NH2
Ester functional group: -COO-
The carboxylic acid functional group is identified by the presence of the -COOH group. It consists of a carbonyl group (C=O) and a hydroxyl group (-OH) attached to the same carbon atom. The carbonyl group is denoted by the C=O bond, and the hydroxyl group is denoted by the -OH. This functional group is responsible for the acidic properties of carboxylic acids, as the hydroxyl group can easily donate a proton (H⁺) in solution. Carboxylic acids have a wide range of applications, including their use as preservatives, flavoring agents, and intermediates in organic synthesis.
The amine functional group is identified by the presence of the -NH₂ group. It consists of a nitrogen atom bonded to two hydrogen atoms (-NH₂). Amines can be classified as primary (1°), secondary (2°), or tertiary (3°) based on the number of carbon groups attached to the nitrogen atom. Amines are basic in nature, as they can accept a proton (H⁺) to form a positively charged ammonium ion. They play important roles in biological processes, such as acting as neurotransmitters, and are also used in the synthesis of various pharmaceuticals, dyes, and polymers.
The ester functional group is identified by the presence of the -COO⁻ group. It consists of a carbonyl group (C=O) attached to an oxygen atom, which is further bonded to a carbon atom (-COO⁻). The carbonyl group is denoted by the C=O bond, and the oxygen atom is denoted by the -O-. Esters are formed through the reaction between a carboxylic acid and an alcohol, resulting in the elimination of water. They are commonly used as solvents, flavoring agents, and fragrances. Esters also play a vital role in the formation of lipids, which are important components of biological membranes and energy storage molecules in organisms.
To learn more about physical and chemical properties, here
https://brainly.com/question/1728902
#SPJ4
What happens to the pressure of a gas in a lightbulb a few minutes after the light is turned on?
Answers
The pressure of a gas in a lightbulb increases a few minutes after the light is turned on.
Pressure is defined as the force exerted by one substance on another per unit area. The force that perhaps the gas applied in such a way on the jar boundaries is defined as gas pressure. Gas molecules move at random across the given volume.
They come into conflict with the surface as well as each other during this movement. Each individual gas molecule's impact is too small and challenging to visualize. However, the combined impact of all gas molecules encompasses the gas pressure.
The larger the number of collisions, the greater the pressure.The average linear momentum of either a gas's moving molecules is then measured by its pressure. The pressure performs perpendicular to the wall constituent of both forces is determined by the viscosity of the gas.
learn more about the pressure of a gas here:
https://brainly.com/question/15265703?referrer=searchResults
#SPJ4
I WILL MARK BRAINEST
SCIENCE
What is the relationship between a need
in society and a system?
Answers
Answer:
The relation between individual and society is very close. Essentially, “society” is the regularities, customs and ground rules of antihuman behavior. These practices are tremendously important to know how humans act and interact with each other. Society does not exist independently without individual.
Explanation:
An ioc occurs when what metric exceeds its normal bounds?.
Answers
An ionic bond is formed when there are more or less electrons that normal in bonding atoms.
What are Ionic bonds?An ionic bond is formed when one atoms donates electrons to another specie to form an ion pair. One atom is positively charged while the other specie is negatively charged.
Hence, an ionic bond is formed when there are more or less electrons that normal in bonding atoms.
Learn more about ionic bonds: https://brainly.com/question/14484184
a pure sample of (r)-limonene has a specific rotation of 125.6. if a mixture of (r)-limonene and (s)-limonene has a specific rotation of 62.8, what are the percentages of the r and s enantiomers in this mixture?
Answers
A pure sample of (r)-limonene has a specific rotation of 125.6. if a mixture of (r)-limonene and (s)-limonene has a specific rotation of 62.8, then the percentage of (s)-limonene is 50.4%.
Given data :
Specific rotation of pure (r)-limonene = 125.6
Specific rotation of mixture of (r)-limonene and (s)-limonene = 62.8
Enantiomers are two stereoisomers that are mirror images of each other and non-superimposable on one another. They have identical physical and chemical properties, except that they rotate plane polarized light in opposite directions.
Each enantiomer rotates the plane of polarization of polarized light in equal amounts but in opposite directions. Therefore, for a mixture of two enantiomers, the observed rotation is the sum of the individual rotations of the two enantiomers.
The percentage of each enantiomer in the mixture can be calculated using the following equation :
percentage of enantiomer = (specific rotation of enantiomer)/(specific rotation of mixture) * 100%
In this case, the percentage of (r)-limonene is 62.8/125.6 * 100% = 49.6%.
Thus, the percentage of (s)-limonene is 100 - 49.6 = 50.4%.
To learn more about enantiomers :
https://brainly.com/question/13265194
#SPJ11
Stickleback fish are collected from a lake in Alaska. 600 from the sample are spineless, which is a recessive trait in the fish. 80 of the fish collected have spines. Calculate:
Answers
Answer:
Frequency of spinal trait in the population = 0.11
Explanation:
Given:
Total spineless trait = 600
Number of spinal trait = 80
Find:
Frequency of spinal trait in the population
Computation:
Total population = Total spineless trait + Number of spinal trait
Total population = 600 + 80
Total population = 680
Frequency of spinal trait in the population = Number of spinal trait / Total population
Frequency of spinal trait in the population = 80 / 680
Frequency of spinal trait in the population = 0.11
While all the other body systems are responsible for keeping you alive, I am responsible for keep your species alive. I am made up of external and internal organs and work differently in males than I do in females. I work very closely with the endocrine system and rely on hormones to maintain homeostasis.
Group of answer choices
Digestive System
Endocrine System
Reproductive System
Immune System
Answers
Answer:
Immune system
Explanation:
There isn't really one, just that the immune system keeps us as a species alive, it prevents spreadable diseases that could wipe out or species
The provision that the seller will release Jacob from any obligation if he loses his job is a(n) _______.
Answers
The provision that the seller will release Jacob from any obligation if he loses his job is a contingency provision.
The provision that the seller will release Jacob from any obligation if he loses his job is a contingency provision. Contingency provisions are clauses included in a contract that specify certain conditions or events that must occur in order for the contract to be fulfilled or terminated. In this case, the contingency provision states that if Jacob loses his job, the seller will release him from any obligation under the contract. It is important to note that contingency provisions must be clearly defined and agreed upon by all parties involved in the contract. This type of provision can help to protect both the buyer and the seller in situations where unexpected circumstances arise. Overall, contingency provisions are a valuable tool for managing risk and ensuring that contractual obligations are met.
To know more about contingency provision visit: https://brainly.com/question/31987975
#SPJ11
what’s the answer to this?
