Answers
Key words:-
Pressure=PVolume=VTemperature=TNow
P1=1atmP_2=0.800atmV1=1.2LV2=LT1=273KT2=26°C=299KApply combined gas law
\(\\ \tt\hookrightarrow \dfrac{P_1V_1}{T_1}=\dfrac{P_2V_2}{T_2}\)
\(\\ \tt\hookrightarrow \dfrac{1(1.2)}{273}=\dfrac{(0.800)V_2}{299}\)
\(\\ \tt\hookrightarrow 0.004=0.002V_2\)
\(\\ \tt\hookrightarrow V_2=2L\)(Approx)
Related Questions
22. Which statement best describes the use of catalytic converters in automobiles?
(1 point)
They decrease the rate of the reactions that produce harmful gases.
They oxidize hydrocarbons to form less toxic gases.
They combine the larger hydrocarbon molecules with smaller ones.
They increase reaction temperature for cleaner burning.
Answers
Answer:
B. They oxidize hydrocarbons to form less toxic gases.
Explanation:
A catalytic converter can be defined as an anti-pollution device containing a catalyst like platinum-iridium, installed in the exhaust chamber of an automobile so as to chemically convert harmful (poisonous) pollutants such as unburned hydrocarbons and carbon monoxide (CO), sulfur dioxide (S02), nitrogen oxide (NO) etc., into less harmful, poisonous or toxic chemical compounds.
This ultimately implies that, catalytic converters are typically used for converting harmful gases into less harmful, poisonous or toxic gases and molecules e.g carbon dioxide (C02) and water (H2O). This helps to prevent global warming, enhance the conservation of natural resources, as well as preserve the lives of living organisms and their natural habitat.
Hence, the statement which best describes the use of catalytic converters in automobiles is that they oxidize hydrocarbons to form less toxic gases.
What is the length of the paper clip in cm
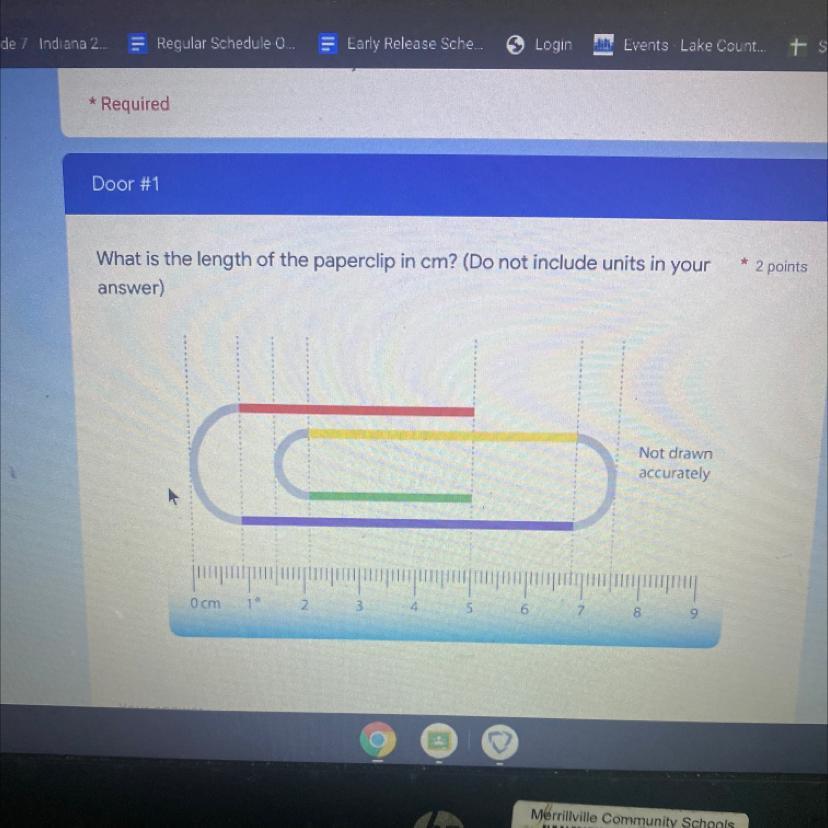
Answers
Answer:
24.5 cm
Explanation:
It would be really complicated to type out, so I've attached an image of how I solved this:
*I separated the paperclip into different sections, figured out the length of those sections, and added them together.
(sorry that my work isn't the neatest)

Milk of magnesia, which is an aqueous suspension of magnesium hydroxide, is used as an antacid in the reaction below. How many molecules of HCl would have to be present to form 34.52 g of MgCl₂?
Mg(OH)₂(s) + 2 HCl(aq) → 2 H₂O(l) + MgCl₂(aq)
Answers
Approximately 4.37 x 10^23 molecules of HCl would be required to form 34.52 g of MgCl₂.
To determine the number of molecules of HCl required to form 34.52 g of MgCl₂, we need to use the molar mass and stoichiometry of the balanced equation:
Mg(OH)₂(s) + 2 HCl(aq) → 2 H₂O(l) + MgCl₂(aq)
The molar mass of MgCl₂ is 95.21 g/mol.
First, we need to calculate the number of moles of MgCl₂ formed:
Moles of MgCl₂ = mass of MgCl₂ / molar mass of MgCl₂
Moles of MgCl₂ = 34.52 g / 95.21 g/mol
Moles of MgCl₂ = 0.363 mol
According to the balanced equation, the stoichiometric ratio between HCl and MgCl₂ is 2:1. Therefore, the moles of HCl required can be calculated as follows:
Moles of HCl = 2 * Moles of MgCl₂
Moles of HCl = 2 * 0.363 mol
Moles of HCl = 0.726 mol
To calculate the number of molecules, we need to use Avogadro's number, which is approximately 6.022 x 10^23 molecules/mol.
Number of molecules of HCl = Moles of HCl * Avogadro's number
Number of molecules of HCl = 0.726 mol * 6.022 x 10^23 molecules/mol
Number of molecules of HCl = 4.37 x 10^23 molecules
Therefore, approximately 4.37 x 10^23 molecules of HCl would be required to form 34.52 g of MgCl₂.
For more such questions on molecules
https://brainly.com/question/1351818
#SPJ8
How many liters of 4.000 M sodium bromide solution are needed to
make 0.250 L of 0.200 M sodium bromide?
Answers
3 ml of urine will make how much of a 1:150 dilution
Answers
3 ml of urine will make a volume of 0.02 ml of a 1:150 dilution.
What is the volume made from the dilution?
To calculate the final volume of a dilution, we need to divide the initial volume by the dilution factor.
Here, we are diluting 3 ml of urine to make a 1:150 dilution. This means that we are diluting 1 part of urine in 150 parts of the diluent.
So, the total volume of the diluted solution will be:
3 ml / 150 = 0.02 ml
Thus, 3 ml of urine will make 0.02 ml of a 1:150 dilution based on the given dilution factor.
Learn more about dilution here: https://brainly.com/question/24881505
#SPJ1
a. 1.7 grams of Ca are mixed with 850.6 ml of 0.043 M HBr. What is the maximum theoretical yield of the gaseous product in grams?
b. how many grams of the excess reagent are left over?
c. what is the pH of the HBr solution?
d. what is the OH- concentration of the HBr solution?
e. if the gas is produced at 89C and 1.7 atm of pressure, what is the volume of gaseous product in mL?
f. the pressure of the gas is changed to 250 mmHg and the volume is changed to 1.54 L. what is the temperature of the gas now?
Answers
A. The maximum theoretical yield of the gaseous product in grams is 0.037 g
B. The grams of the excess reagent are left over is 0.97 g
C. The pH of the HBr solution is 1.37
D. The OH¯ concentration of the HBr solution is 2.33×10¯¹³ M
E. The volume (in mL) of the gaseous product is 323 mL
F. The new temperature of the gas is 61 °C
How to determine the mass of HBrWe'll begin by calculating the mole of HBr in the solution. This is illustrated below:
Volume = 850 mL = 850.6 / 1000 = 0.8506 L Molarity = 0.043 MMole of HBr =?Molarity = mole / Volume
Mole = molarity × volume
Mole of HBr = 0.043 × 0.8506
Mole of HBr = 0.0366 mole
Thus, the mass of HBr can be obtained as follow:
Mole of HBr = 0.0366 moleMolar mass of HBr = 81 g/molMass of HBr =?Mass = mole × molar mass
Mass of HBr = 0.0366 × 81
Mass of HBr = 2.96 g
A. How to determine the maximum theoretical yieldBalanced equation
Ca + 2HBr --> CaBr₂ + H₂
Molar mass of Ca = 40 g/mol
Mass of Ca from the balanced equation = 1 × 40 = 40 g
Molar mass of HBr = 81 g/mol
Mass of HBr from the balanced equation = 2 × 81 = 162 g
Molar mass of H₂ = 2 g/mol
Mass of H₂ from the balanced equation = 1 × 2 = 2 g
SUMMARY
From the balanced equation above,
40 g of Ca reacted with 162 g of HBr to produce 2 g of H₂
Next, we shall determine the limiting reactant.
From the balanced equation above,
40 g of Ca reacted with 162 g of HBr.
Therefore,
1.7 g of Ca will react with = (1.7 × 162) / 40 = 6.885 g of HBr.
Since a higher amount of HBr is needed, therefore HBr is the limiting reactant and Ca is the excess reactant
Finally, we shall determine the maximum theoretical yield of the gaseous product. details below
From the balanced equation above,
162 g of HBr reacted to produce 2 g of H₂.
Therefore,
2.96 g of HBr will react to produce = (2.96 × 2) / 162 = 0.037 g of H₂
Thus, The maximum theoretical yield of the gaseous product obtained is 0.037 g
B. How to determine the mass of the excess reactant leftoverCa is the excess reactant
From the balanced equation above,
162 g of HBr reacted with 40 g of Ca.
Therefore,
2.96 g of HBr will react with = (2.96 × 40) / 162 = 0.73 g
Thus, the mass of the excess reactant leftover can be obtained as illustrated below:
Mass of excess reactant given = 1.7 gMass of excess reactant that reacted = 0.73 gMass of excess reactant leftover =?Mass of excess reactant leftover = 1.7 - 0.73
Mass of excess reactant leftover = 0.97 g
C. How to determine the pH of HBrMolarity of HBr = 0.043 MHydrogen ion concentration [H⁺] = 0.043 MpH =?pH = –Log H⁺
pH = –Log 0.043
pH = 1.37
D. How to determine the OH¯ concentrationHydrogen ion concentration [H⁺] = 0.043Hydroxide ion concentration [OH¯] =?[H⁺] × [OH¯] = 10¯¹⁴
0.043 × [OH¯] = 10¯¹⁴
Divide both side by 0.043
[OH¯] = 10¯¹⁴ / 0.043
[OH¯] = 2.33×10¯¹³ M
E. How to determine the volume of the gas productTemperature (T) = 89 °C = 89 + 273 = 362 KPressure (P) = 1.7 atmGas constant (R) = 0.0821 atm.L/Kmol Mass of gas product (H₂) = 0.037 g Molar mass of H₂ = 2 g/molNumber of mole (n) = 0.037 / 2 = 0.0185 moleVolume (V) =?Using the ideal gas equation, the volume of the gas can be obtained as follow:
PV = nRT
Divide both side by P
V = nRT / P
V = (0.0185 × 0.0821 × 362) / 1.7
V = 0.323 L
Multiply by 1000 to express in mL
V = 0.323 × 1000
V = 323 mL
F. How to determine the new temperatureInitial volume (V₁) = 323 mL = 323 / 1000 = 0.323 LInitial pressure (P₁) = 1.7 atmInitial temperature (T₁) = 89 °C = 89 + 273 = 362 KNew Volume (V₂) = 1.54 L New pressure (P₂) = 250 mmHg = 250 / 760 = 0.329 atmNew temperature (T₂) =?The new temperature of the gas can be obtained by using the combined gas equation as illustrated below:
P₁V₁ / T₁ = P₂V₂ / T₂
(1.7 × 0.323) / 362 = (0.329 × 1.54) / T₂
Cross multiply
1.7 × 0.323 × T₂ = 362 × 0.329 × 1.54
Divide both side by 1.7 × 0.323
T₂ = (362 × 0.329 × 1.54) / (1.7 × 0.323 )
T₂ = 334 K
Subtract 273 to obtain answer in °C
T₂ = 334 – 273 K
T₂ = 61 °C
Learn more about molarity:
https://brainly.com/question/9468209
Learn more about stoichiometry:
https://brainly.com/question/14735801
Learn more about pH:
https://brainly.com/question/3709867
Learn more about ideal gas equation:
https://brainly.com/question/4147359
Learn more about gas laws:
https://brainly.com/question/6844441
#SPJ1
A metal carbonate, XCO3 of mass 2.012 g was heated resulting in the formation of XO, a metal oxide and carbon dioxide with a mass of 0.855 g according to the reaction shown below: XCO3 (s) → XO (s) + CO2 (g) (Atomic mass of O-15.999 g/mol; H-1.008 g/mol; C-12.011 g/mol).
Answers
The metal X has an approximate molar mass of 42.36 g/mol and the metal is most likely calcium.
What is the molar mass of XCO₃?The molar mass of the metal carbonate XCO₃ and identify the metal X, we need to calculate the number of moles of XCO₃ and CO₂ using the given masses and molar masses.
The molar mass of CO₂ (carbon dioxide) is 12.011 g/mol (for carbon) + 2 * 15.999 g/mol (for oxygen) = 44.01 g/mol.
The number of moles of CO₂ can be calculated using the formula:
moles of CO₂ = mass of CO₂ / molar mass of CO₂
moles of CO₂ = 0.855 g / 44.01 g/mol
moles of CO₂ ≈ 0.01944 mol
Since the reaction stoichiometry is 1:1 between XCO₃ and CO₂, the number of moles of XCO₃ is also approximately 0.01944 mol.
molar mass of XCO₃ = mass of XCO₃ / moles of XCO₃
molar mass of XCO₃ = 2.012 g / 0.01944 mol
molar mass of XCO₃ ≈ 103.38 g/mol
The molar mass of XCO₃ is approximately 103.38 g/mol.
To determine the metal X:
molar mass of X = molar mass of XCO3 - molar mass of CO3
molar mass of X = 103.38 g/mol - (12.011 g/mol + 3 * 15.999 g/mol)
molar mass of X ≈ 42.36 g/mol
Metal X is most likely Calcium that has a molar mass of 40 g/mol
Learn more about molar mass at: https://brainly.com/question/837939
#SPJ1
How does matter and energy cycle through an ecosystem?
a.
Cellular respiration alone
b.
The atmosphere
c.
Photosynthesis and cellular respiration
d.
Photosynthesis alone
Please help D;
Answers
Answer:
C
Explanation:
Determine if the following salt is neutral, acidic or basic. If acidic or basic, write the appropriate equilibrium equation for the acid or base that exists when the salt is dissolved in aqueous solution. If neutral, write only NR. LiNO₃
Answers
Answer:
It is neutral (NR)
Explanation:
Salts are formed when the ionizable hydrogens in an acid is replaced by metallic or ammonium ions from bases. The reaction is known as a neutralization reaction.
The nature of a salt formed from this reaction depends on the nature of the reacting acid and base.
If the reaction is between a strong acid and strong base, the salt produced is a neutral salt.
If the reaction occurs between a strong acid and a weak base, the salt produced is acidic.
If the reaction occurs between a strong base and a weak acid, the salt produced is a basic salt.
Considering the salt above, LiNO3.
On hydrolysis, addition of water, the following products are obtained:
LiNO3 + H2O ----> LiOH + HNO3
The products obtained, LiOH and HNO3 are a strong base and a strong acid respectively. Therefore, the salt, LiNO3, is a neutral salt.
The salt, LiNO₃ is a neutral, NR salt as it's a salt formed from the reaction of a strong acid and a strong base.
In neutralisation reactions, acids and bases react to form salt and water.
However, the salt formed may be acidic, basic or neutral. This is dependent on the type of acid and base which form the salt.
A strong acid and a strong base react to yield a neutral salt like, LiNO₃.
The equilibrium equation when LiNO₃ is dissolved in aqeous solution is;
LiNO₃ + H2O ==>. LiOH + HNO₃Evidently, LiOH and HNO₃ are an example strong base and acid respectively.
Read more:
https://brainly.com/question/2254059
If you have 25 moles of water, H2O, how many molecules of water do you have?
Answers
Answer:
The number of molecules of water us 1.50× 10²⁵ molecules
Explanation:
From N=nL
where L =avogadro number ( 6.02× 10^²³ entities)
The number of the molecules of water =1
n (amount of substance)=25 moles
hence (N) = 25×1×6.02×10^²³
=1.50×10²⁵ molecules of H2O
The empirical formula for a compound that contains 6.34 grams carbon and 1.06 grams hydrogen is
Answers
Answer:
\(CH_2\)Explanation:
Here, we want to get an empirical formula
We start by dividing each of the masses by the corresponding atomic masses
The atomic mass of carbon is 12 amu
The atomic mass of hydrogen is 1 amu
We start the division as follows:
\(\begin{gathered} C\text{ = }\frac{6.34}{12}\text{ = 0.5283333} \\ \\ H\text{ = }\frac{1.06}{1}\text{ = 1.06} \end{gathered}\)Now, we divide the results by the smaller of the two:
\(\begin{gathered} C\text{ = }\frac{0.528333}{0.529333}\text{ = 1} \\ \\ H\text{ = }\frac{1.06}{0.528333}\text{ = 2} \end{gathered}\)Thus, we have the empirical formula as:
\(CH_2\)Write a chemical equation representing the second ionization energy for lithium. Use e− as the symbol for an electron.
Answers
Answer: Li^+ (g) ----> Li^2+ (g) + e^-
Considering the definition of first and second ionization energy, the second ionization energy of lithium is:
Li⁺ → Li²⁺ + 1 e-
In first place, the electrons are attracted to the nucleus and it is necessary to provide energy to start them. Ionization energy is the energy required to remove an electron from a gaseous atom, isolated and in a ground state. The electrons in the last shell, which are the weakest attracted to the nucleus, are always lost. In this way the neutral atom becomes a gaseous cation (ion with a positive charge).
So, in this case, the first ionization energy of lithium is:
Li → Li⁺ + 1 e-
The energy required to remove a second electron is called the second ionization energy and due to the difficulty of removing an electron from a positive particle, its value is greater than the first ionization energy (the volume of a positive ion is less than that of the neutral atom and the electrostatic force is greater in the positive ion than in the atom)
Finally, in this case, the second ionization energy of lithium is:
Li⁺ → Li²⁺ + 1 e-
Learn more:
brainly.com/question/16243729?referrer=searchResults brainly.com/question/11623163?referrer=searchResults brainly.com/question/1602374?referrer=searchResults
which of the following describes an experimental technology being used to reduce carbon dioxide emissions from coal?
Answers
Carbon capture and storage is one experimental method being utilised to lower carbon dioxide emissions from coal (CCS).
One experimental technique being used to reduce carbon dioxide emissions from coal is carbon capture and storage (CCS). With CCS, carbon dioxide emissions from factories or power plants are captured and either stored underground in geological formations or used to improve oil recovery.
Coal and other fossil fuels have the potential to drastically cut their carbon dioxide emissions, but CCS technology currently in the experimental stage. Unfortunately, because of its expensive cost and technical implementation difficulties, the technology is not yet extensively employed. In order to address the current climate problem, efforts to cut CO2 emissions are essential.
Learn more about carbon dioxide here:
https://brainly.com/question/9419166
#SPJ4
What experimental technology is being used to reduce carbon dioxide emissions from coal?
How many grams of sodium chloride should you theoretically produce if you start with 5.00 grams of calcium chloride and excess sodium carbonate? (answer in numbers only - no units or words)

Answers
Answer:
5.27 g of NaCl
Explanation:
The balanced equation for the reaction is given below:
Na₂CO₃ + CaCl₂ —> 2NaCl + CaCO₃
Next, we shall determine the mass of CaCl₂ that reacted and the mass of NaCl produced from the balanced equation. This can be obtained as follow:
Molar mass of CaCl₂ = 40 + (35.5×2)
= 40 + 71
= 111 g/mol
Mass of CaCl₂ from the balanced equation = 1 × 111 = 111 g
Molar mass of NaCl = 23 + 35.5
= 58.5 g/mol
Mass of NaCl from the balanced equation = 2 × 58.5 = 117 g
Summary:
From the balanced equation above,
111 g of CaCl₂ reacted to produce 117 g of NaCl.
Finally, we shall determine the theoretical yield of NaCl. This can be obtained as follow:
From the balanced equation above,
111 g of CaCl₂ reacted to produce 117 g of NaCl.
Therefore, 5 g of CaCl₂ will react to produce = (5 × 117)/111 = 5.27 g of NaCl.
Thus, the theoretical yield of NaCl is 5.27 g.
Which of the following set of quantum numbers (ordered n, ℓ, mℓ ) are possible for an electron in an atom? Check all that apply
a. 2, 1, 3
b. 5, 3, -3
c. 4, 3, -2
d. -4, 3, 1
e. 2, 1, -2
f. 3, 2, 2
g. 3, 3, 1
Answers
the possible quantum numbers (ordered n, ℓ, mℓ ) are:Option B.5, 3, -3 and Option C. 4, 3, -2
The quantum numbers n, ℓ, mℓ represent respectively the principal quantum number, the orbital angular momentum quantum number and the magnetic quantum number.
These are the three most important quantum numbers. T
here is another quantum number called the spin quantum number, denoted by ms.
Let's see which of the given quantum number sets is possible.2, 1, 3 is not possible because for ℓ = 1, mℓ can only be -1, 0, or 1. 5, 3, -3 is possible.4, 3, -2 is possible. -4, 3, 1 is not possible.
For any value of ℓ, mℓ must be between -ℓ and +ℓ. e. 2, 1, -2 is not possible because for ℓ = 1, mℓ can only be -1, 0, or 1. f. 3, 2, 2 is not possible because for ℓ = 2, mℓ can only be -2, -1, 0, +1, or +2. g. 3, 3, 1 is not possible because for any value of ℓ, mℓ must be between -ℓ and +ℓ.
Therefore, the possible quantum numbers (ordered n, ℓ, mℓ ) are:5, 3, -34, 3, -2
For more questions on quantum numbers
https://brainly.com/question/30881398
#SPJ8
in which beakers are the particles moving the most slowly
Answers
Answer:
A and D
Explanation:
2NH3(g)−→−−−−catalystN2(g)+3H2(g) On the basis of the data in the graph, which of the following best represents the rate law for the catalyzed decomposition of NH3(g)?
Answers
The best represents the rate law for the catalyzed decomposition of NH3(g) rate=k.
What is meant by catalyzed ?
acting as a catalyst to cause a chemical reaction to occur or to progress more quickly: It was once believed that cells' processes could only be catalyzed by proteins.Catalysis is the process by which the addition of a substance (the catalyst) that is not itself modified during the chemical reaction changes the rate at which chemical reactions occur.Usually, catalyzed reactions are employed to quicken the rate at which a certain chemistry develops. The catalyst's main function is to give the reaction a another, lower-energy pathway.A substance that initiates or accelerates a chemical reaction without changing itself is known as a catalyst.A circumstance, action, or someone responsible for a significant shift.To learn more about catalyzed refer to
https://brainly.com/question/12507566
#SPJ4
Calculate the approximate number of sodium ions in 2.52 g of sodium sulphate,
Na2SO4.
Answers
Explanation:
Please send me the answer of this question
Calculate the mass percent of .485g of H, which reacts with O to form 2.32g H2O?
Answers
Answer:
53.1% of hydrogen reacts
Explanation:
The mixture of 2 atoms of H with 1 atom of O produce 1 molecule of H₂.
The mass of hydrogen in 2.32g of H₂O could be obtained using molar mass of H₂O (18.01g/mol) and molar mass of hydrogen (1.01g/mol) as follows:
Moles H₂O: 2.32g H₂O × (1mole / 18.01g) = 0.1288 moles of water
1 mole of H₂O contains 2 moles of H, moles of hydrogen in 0.1288 moles of water are:
0.1288 moles H₂O × (2 moles H / 1 mole H₂O) = 0.2576 moles of H
In mass:
0.2576 moles H × (1.01g/ mol H) = 0.260g H you have in the formed water
As before reaction you had 0.485g of H and just 0.260g reacted, mass percent is:
(Mass that reacts / Mass added) × 100
(0.260g / 0.485g) × 100 =
53.1% of hydrogen reactshow to synthesize 2-benzyl pentanoic acid from acetoacetic ester?
Answers
If you're attempting to synthesize 2 benzyl pentanoic acid from acetoacetic ester, keep in mind that you can do so fairly quickly by following these simplified instructions:
Begin by dissolving your acetoacetic ester into anhydrous diethyl ether and adding benzyl bromide and sodium hydroxide to the mix. Stir it all together at room temperature for around thirty minutes before reacting it with hydrochloric acid so that any remaining solvent evaporates out of your crude mixture; Lastly refine your creation by recrystallizing it from ethanol until you have pure 2 benzyl pentanoic acid.What is acetoacetic ester?From its pungent free scent to its solid state at temperatures ranging from 118 120°C, acetoacetic ester (better known as ethyl acetoacetate) offers significant value for those working within organic synthesis.
As one of many potent ketones utilized by researchers around the globe its unique properties make it ideal for building complex molecules essential for modern medicine and more.
Learn about acetoacetic ester here https://brainly.com/question/31744037
#SPJ1
985.2 moles of nitrogen, how many moles of ammonia can produce?
Answers
Answer:
985.2 moles of nitrogen can produce 1970.4 moles of ammonia.
Explanation:
The balanced chemical equation for the production of ammonia from nitrogen is:
N2 + 3H2 → 2NH3
From the balanced equation, we can see that 1 mole of nitrogen reacts with 3 moles of hydrogen to produce 2 moles of ammonia.
So, to determine how many moles of ammonia can be produced from 985.2 moles of nitrogen, we need to use the mole ratio from the balanced chemical equation as follows:
985.2 moles N2 x (2 moles NH3 / 1 mole N2) = 1970.4 moles NH3
Therefore, 985.2 moles of nitrogen can produce 1970.4 moles of ammonia.
You have 3L of a 2 molar magnesium chloride solution. How many moles of chloride ions are present?
Show all work!
Answers
Answer:
12 moles Cl⁻
Explanation:
To find the moles Cl⁻, you need to (1) calculate the moles of magnesium chloride (MgCl₂) using the molarity equation and then (2) convert moles MgCl₂ to moles Cl⁻ (via the mole-to-mole ratio from formula subscripts). It is important to arrange the conversions in a way that allows for the cancellation of units.
Molarity (M) = moles / volume (L)
2 M = moles / 3 L
6 = moles MgCl₂
1 MgCl₂ = 1 Mg²⁺ and 2 Cl⁻
6 moles MgCl₂ 2 moles Cl⁻
------------------------ x ----------------------- = 12 moles Cl⁻
1 mole MgCl₂
1)You are given an unknown solution, which contains both aqueous barium ion, Ba2+ (aq), and aqueous lead(II)ion, Pb2+ (aq). Which of
your six solutions would allow separation of the ions
Answers
If you look at the six solutions, ammonium chloride is the one that will separate barium ion from lead (II) ion in water.
If you want to separate things in a solution, you can use a solution that preferentially precipitates one of the ions in the solution while leaving the other one in the solution.
Here, we know that chlorides are soluble except those that come from metals like gold and silver because of the rules about which ones can be soluble. This is why, when barium ion and lead (II) chloride are mixed together in water, lead II chloride preferentially precipitates. This means the ions are separated.
I hope this helps you
:)
An unknown solution, which contains both aqueous barium ion, Ba2+ (aq), and aqueous lead(II)ion, Pb2+ (aq). Therefore, option A, D and E are correct.
What is solubility ?The maximum quantity of a chemical that will dissolve in a given amount of solvent at a particular temperature is referred to as its solubility. Different compounds have very varying solubilities, which is a feature of a particular solute-solvent pair.
According to solubility rules
1.Li+, Na+, K+, NH4+ always soluble .
2.NO3-, C2H3O2- always soluble .
3.Cl- , Br-, I- insoluble with Ag+, Hg22+, Pb2+ but soluble with any other ions.
4.SO4- soluble with all the ions except Sr2+, Ba2+, Ca2+, Ag+, Hg22+, or Pb2+.
5. CO32-, PO43- soluble with Li+ , Na+, K+, NH4+ but insoluble with any other ion.
6.OH- , S2- soluble with Ca2+ , Sr2+ , Ba2+ , Li+ , Na+ , K+ , NH4+ but insoluble with any other ions.
In NH4Cl, cl- ions make precipitate with Pb2+ but not with Ba2+.So, they are separated.
In NaCl, cl- ions make precipitate with Pb2+ but not with Ba2+. So, they are separated.
In NaOH, OH- ions make precipitate with Pb2+ but not with Ba2+.So, they are separated.
Thus, option A, D and E are correct.
To learn more about the solubility, follow the link;
https://brainly.com/question/29661360
#SPJ2
Your question is incomplete, most probably your question is attached below in image.

HELP ASAP!!!!!!!!!!
What is sous-vide?
A food stabilizer
B liquid food that chefs make into a spherical shape
C food that chefs quickly freeze using liquid nitrogen
D food that chefs seal in a plastic bag and cook under controlled temperature conditions
Answers
Answer:
D) food that a chef seal in a plastic bag and cook under a controlled temperature condition.
Explanation:
Sous vide, also known as low temperature long time cooking, is a method of cooking in which food is placed in a plastic pouch or a glass jar and cooked in a water bath for longer than usual cooking times at a precisely regulated temperature.
How many grams of sodium bromide must be dissolved in 500.0 g of water to produce a 0.600 molal solution?
Answers
Molality is a measure of concentration and it is defined as the moles of solute dissolved in 1 kg of solvent.
molality = moles of solute/(mass of solvent in kg)
We are given the molal concentration of the solution and the mass of solvent (water), using the formula we can find the number of moles of sodium bromide.
molality = 0.600 m
1000 g = 1 kg
mass of solvent = 500.0 g * 1 kg/(1000 g)
mass of solvent = 0.500 kg
molality = moles of solute/(mass of solvent in kg)
moles of solute = molality * mass of solvent
moles of solute = 0.600 m * 0.500 kg
moles of solute = 0.300 moles
We found that we have to dissolve 0.300 moles of sodium bromide in 0.500 kg of water to get a 0.600 molal solution. We are asked for the mass, so we can use the molar mass of sodium bromide to get it.
formula of sodium bromide = NaBr
atomic mass of Na = 22.99 amu
atomic mass of Br = 79.90 amu
molar mass of NaBr = 22.99 + 79.90
molar mass of NaBr = 102.89 g/mol
mass of NaBr = moles of NaBr * molar mass of NaBr
mass of NaBr = 0.300 moles * 102.89 g/mol
mass of NaBr = 30.9 g
Answer: we must dissolve 30.9 g of sodium bromide.
6. How many moles are in 8.30 x 1023 molecules of CO₂?
a.
b.
C.
d.
1.37
2.8
55.5
100
Answers
A 0.231 M solution of acetate has a pOH of 4.90. What is the Kb of acetate?
Answers
Explanation:
o solve this problem, we need to use the relation between pOH, pH, and the dissociation constant of the conjugate base of the weak acid, which is given by:
Kb = Kw / Ka
where Kb is the dissociation constant of the conjugate base, Ka is the dissociation constant of the weak acid, and Kw is the ion product constant of water, which is 1.0 x 10^-14 at 25°C.
First, we need to find the pH of the solution, since we know the pOH:
pH + pOH = 14
pH = 14 - 4.90 = 9.10
The weak acid in this case is the acetic acid (CH3COOH), which dissociates in water according to the equation:
CH3COOH + H2O ↔ CH3COO- + H3O+
The dissociation constant of acetic acid (Ka) is 1.8 x 10^-5 at 25°C. We can use this value and the relation between Ka and Kb to find Kb:
Kb = Kw / Ka = 1.0 x 10^-14 / 1.8 x 10^-5 = 5.56 x 10^-10
Therefore, the Kb of acetate is 5.56 x 10^-10.
Ammonium nitrate has been used as a high explosive because it is unstable and decomposes into several gaseous substances. The rapid expansion of the gaseous substances produces the explosive force.
NH4NO3(s) → N2(g) + O2(g) + H2O(g)
Calculate the mass of each product gas if 1.29 g of ammonium nitrate reacts.
N2
O2
H2O
Answers
1. The mass of N₂ produced is 0.45 g
2. The mass of O₂ produced is 0.26 g
3. The mass of H₂O produced is 0.58 g
Balanced equation4NH₄NO₃(g) --> 4N₂(g) + 2O₂(g) + 8H₂O(g)
Molar mass of NH₄NO₃ = 80 g/mol
Mass of NH₄NO₃ from the balanced equation = 4 × 80 = 320 g
Molar mass of N₂ = 28 g/mol
Mass of N₂ from the balanced equation = 4 × 28 = 112 g
Molar mass of O₂ = 32 g/mol
Mass of O₂ from the balanced equation = 2 × 32 = 64 g
Molar mass of H₂O = 18 g/mol
Mass of H₂O from the balanced equation = 8 × 18 = 144 g
SUMMARY
From the balanced equation above,
320 g of NH₄NO₃ decomposed to produce 112 g of N₂, 64 g of O₂ and 144 g of H₂O
1. How to determine the mass of N₂ producedFrom the balanced equation above,
320 g of NH₄NO₃ decomposed to produce 112 g of N₂
Therefore,
1.29 g of NH₄NO₃ will decompose to produce = (1.29 × 112) / 320 = 0.45 g of N₂
2. How to determine the mass of O₂ producedFrom the balanced equation above,
320 g of NH₄NO₃ decomposed to produce 64 g of O₂
Therefore,
1.29 g of NH₄NO₃ will decompose to produce = (1.29 × 64) / 320 = 0.26 g of O₂
3. How to determine the mass of H₂O producedFrom the balanced equation above,
320 g of NH₄NO₃ decomposed to produce 144 g of H₂O
Therefore,
1.29 g of NH₄NO₃ will decompose to produce = (1.29 × 144) / 320 = 0.58 g of H₂O
Learn more about stoichiometry:
https://brainly.com/question/14735801
#SPJ1
What type of marks might link the bullet to a specific weapon? Which type of fingerprints has she found?
Answers
Marks that might link a bullet to a specific weapon include striations, which are unique patterns and imperfections left on the bullet's surface as it passes through the barrel of a firearm. As for the fingerprints found, it depends on the context of the investigation. Fingerprints can be categorized into three main types: latent, patent, and plastic.
Marks that might link a bullet to a specific weapon include striations, which are unique patterns and imperfections left on the bullet's surface as it passes through the barrel of a firearm. These striations are caused by the unique characteristics of the rifling inside the barrel, such as the number of lands and grooves, their width, depth, and the direction of twist. Comparing the striations on a recovered bullet to test-fired bullets from a suspected weapon can help determine if they match, indicating a potential link.
As for the fingerprints found, it depends on the context of the investigation. Fingerprints can be categorized into three main types: latent, patent, and plastic. Latent fingerprints are invisible and require development techniques such as powder or chemical methods to make them visible. Patent fingerprints are visible prints left on a surface, such as blood or dirt. Plastic fingerprints are three-dimensional impressions left on soft surfaces like clay or wax.
Without specific information on the crime scene or the type of fingerprints found, it is not possible to determine which type the investigator has encountered.
For more question on striations
https://brainly.com/question/20980710
#SPJ8
you are studying a protein that is translocated when activated and results in uncontrolled proliferation. please design a primary high throughput assay, orthogonal assay, and a validation experiment to identify a drug that can inhibit this target.
Answers
The activation of the protein leads to uncontrolled proliferation through a complex cellular signaling cascade. Upon activation, the protein is translocated to the nucleus where it binds to specific DNA sequences and alters gene expression.
This results in the production of proteins that stimulate cell growth and division. In addition, the activated protein can also affect signaling pathways that control the cell cycle, leading to uncontrolled cell division. Overexpression or mutation of the protein can lead to dysregulation of these signaling pathways and contribute to the development of cancer. Understanding the molecular mechanisms of protein activation and its downstream effects can inform the development of targeted cancer therapies.
To know more about DNA sequences, here
brainly.com/question/30590319
#SPJ1
--The complete Question is, What is the mechanism by which the activation of the protein leads to uncontrolled proliferation?--