a monolayer containing 3.18×10-6 g of oleic acid has an area of 20.0 cm2. the density of oleic acid is 0.895 g / ml. what is the thickness of the monolayer (the length of an oleic acid molecule)? 5.69×10-5 cm 1.42×10-7 cm 7.11×10-5 cm 5.63×10-6 cm 1.78×10-7 cm
Answers
A monolayer is containing 3.18×10-6 g of oleic acid has an area of 20.0 cm2, thus the thickness of the monolayer is calculated as 1.777 × 10⁻⁷ cm
Thickness of MonolayerUsing,
Density = Mass / Volume
It is given that:
Area = 20.0 cm²
Density = 0.895 g/mL
Mass = 3.18 × 10⁻⁶ g
Then, applying the density formula as:
0.895 g/mL = 3.18 × 10⁻⁶ g / Volume (ml)
Volume = 3.55 × 10⁻⁶ mL
Since, 1 mL = cm³
So, Volume = 3.55 × 10⁻⁶ cm³
Thickness of monolayer = ?
Also, Volume = area x thickness
So, then we have
Thickness = Volume/Area = 3.55 × 10⁻⁶ cm³ / 20.0 cm²
= 1.777 × 10⁻⁷ cm
To know more about monolayer, visit:
https://brainly.com/question/14699941
#SPJ4
Related Questions
what is a correct set of quantum numbers for an electron in the 3p orbital? fill in the blanks with a whole number no decimals n
Answers
\(\begin{equation}\mathrm{n}=3, \rho=1, \mathrm{m_e}=-1,0, \mathrm{~m}_{\mathrm{s}}=+1 / 2 \text { or }-1 / 2\end{equation}\) is a correct set of quantum numbers for an electron in the 3p orbital, with a whole number no decimals n.
The quantum state of an electron in an atom or molecule is determined by four separate elements known as quantum numbers. With the use of these numbers, it is possible to forecast the energy, location, and orientation of the electrons within an atom. The orbital's size is defined by the electron's principal quantum number, n, which also serves as an energy level indicator. The azimuthal quantum number, l, describes the geometry of the orbital, while the magnetic quantum number, ml, determines its direction in space. An electron's spin is defined by its spin quantum number, or ms. These illustrations are crucial for understanding atomic and molecular properties since they collectively give a full explanation of the electron.
Learn more about quantum numbers here:
brainly.com/question/19346628
#SPJ4
The actual question is:
Fill in the blanks,
__________________ is a correct set of quantum numbers for an electron in the 3p orbital, with a whole number no decimals n
A) \(\begin{equation}\mathrm{n}=3, \rho=1, \mathrm{m_e}=-1,0, \mathrm{~m}_{\mathrm{s}}=+1 / 2 \text { or }-1 / 2\end{equation}\)
B) \(\begin{equation}\mathrm{n}=3, \rho=2, \mathrm{m_e}=-2,0, \mathrm{~m}_{\mathrm{s}}=+1 / 2 \text { or }-1 / 2\end{equation}\)
C) \(\begin{equation}\mathrm{n}=2, \rho=2, \mathrm{m_e}=-1,0, \mathrm{~m}_{\mathrm{s}}=+1 / 2 \text { or }-1 / 2\end{equation}\)
D) \(\begin{equation}\mathrm{n}=2, \rho=3, \mathrm{m_e}=-1,0, \mathrm{~m}_{\mathrm{s}}=+1 / 2 \text { or }-1 / 2\end{equation}\)
a beaker containing 50.0 ml of 0.50 m naoh is titrated using a burette containing a solution of 0.50 m hcl. report all answers to two decimal places (e.g. 1.00) prompt 1what is the resulting ph of the solution if only 25.0 ml of titrant is added? answer for prompt 1 what is the resulting ph of the solution if only 25.0 ml of titrant is added? prompt 2what is the resulting ph of the solution if 50.0 ml of titrant is added? answer for prompt 2 what is the resulting ph of the solution if 50.0 ml of titrant is added? prompt 3what is the resulting ph of the solution if 75.0 ml of titrant is added?
Answers
The pH of the solution after 28.0 ml of NaOH have been added to the acid exists 0.85.
How to estimate the ph of the solution?The balanced reaction between base and acid exists;
NaOH + HCl → NaCl + H₂O
NaOH exists a strong base and HCl exists a strong acid therefore complete dissociation. Stoichiometry of acid to base exists 1: 1.
The number of moles of base added - 0.5 M /1000 mL/L × 28.0 mL = 0.014 mol
the number of acid moles present - 0.5 M /1000 mL/L × 50.0 mL = 0.025 mol.
acid reacts with base 1: 1 ratio
Therefore excess amount of acid present - 0.025 - 0.014 = 0.011 mol
Total volume = 50.0 + 28.0 = 78.0 mL
[H⁺] = 0.011 mol/0.078 L = 0.14 M
Therefore, pH = -log [H⁺]
substitute the values in the above equation, we get
pH = -log(0.14)
simplifying the above equation, we get
pH = 0.85.
Therefore, the pH of the solution after 28.0 ml of NaOH have been added to the acid exists 0.85.
To learn more about pH values refer to:
https://brainly.com/question/172153
#SPJ4
True or false; A solution always contains only one solvent.
Answers
A solution is defined as a mixture of two or more substances, usually, a solute and a solvent, and the difference between these two are in quantity, solute represents the smallest amount and solvent will represent the highest amount, and while you can have more than one solute, you can only have one solvent for a solution. Therefore the statement is true
A stick of butter is melted in a saucepan. As it continues to cook, the butter turns brown. What changes have occurred?
Answers
Answer:
Assuming that the butter is already a liquid, it is a chemical change in which the milk solids of the butter oxidize.
Explanation:
I hope this helps :)
Chemical changes have occurred as the stick of butter melts as the milk solids present in butter undergo oxidation resulting in formation of new substances.
What are chemical changes?Chemical changes are defined as changes which occur when a substance combines with another substance to form a new substance.Alternatively, when a substance breaks down or decomposes to give new substances it is also considered to be a chemical change.
There are several characteristics of chemical changes like change in color, change in state , change in odor and change in composition . During chemical change there is also formation of precipitate an insoluble mass of substance or even evolution of gases.
There are three types of chemical changes:
1) inorganic changes
2)organic changes
3) biochemical changes
During chemical changes atoms are rearranged and changes are accompanied by an energy change as new substances are formed.
Learn more about chemical changes,here:
https://brainly.com/question/23693316
#SPJ5
1. The forks shown are made of silver (Ag). Some of the silver forks
shown have lost their luster—they have become tarnished. This is an
example of Type here.
Pleasee help
Answers
Answer:
Corrosion
Explanation:
Silver is known as a noble metal. Noble metals are those metals that are resistant to corrosion.
However, we can not say that silver is completely resistant to corrosion because silver can corrode when exposed to air and sulphur.
Exposure to air and sulphur leads to the coating of the silver with a sulphide layer thereby making the metal to become tarnished and loose its metallic luster.
Ca is an atom. Why?
Help me asap pleased
Answers
Answer:
It has protons, neutrons and electrons
The addition of solute particles into a solution causes:
A. the solvent particles to be attracted to the solute particles and to have less kinetic energy.
B. the solute particles to bump the solvent particles out of the solution, making it easier to boil.
C. the solute particles to form seed crystals and make the solution freeze more easily.
D. the solvent particles to be spread further out and have more to move, increasing their kinetic energy.
Answers
Answer:
D. the solvent particles to be spread further out and have more to move, increasing their kinetic energy.
Explanation:
The addition of solute particles results in an increased boiling point. As the solutes increases so does the energy inside as the solutes add and occupies more space near the surface of the liquid.I added 6 to a certain number and then divide the result by 3. Find the number if my final answer is 5. (show working)
Answers
Answer:
let the number be x
so (x+6)/3 =5
x+6=15
x=9
When is a rock considered an ore?
Check all that apply.
-when it occurs in sufficient amounts to be used to build roads and buildings
-only when it contains lead
-when it contains at least one metallic mineral in sufficient amounts to be extracted profitably
-only when it contains iron
-when it contains fluorite or sulfur minerals in sufficient amounts to be extracted profitably
Answers
The correct statements are that a rock is considered an ore when it contains at least one metallic mineral in sufficient amounts to be extracted profitably and when it contains fluorite or sulfur minerals in sufficient amounts to be extracted profitably.
When it contains at least one metallic mineral in sufficient amounts to be extracted profitably.
When it contains fluorite or sulfur minerals in sufficient amounts to be extracted profitably.
Step-by-step explanation:
When it occurs in sufficient amounts to be used to build roads and buildings: This statement is incorrect. Rocks that are used for construction purposes, such as limestone or sandstone, are not considered ores unless they also contain economically valuable minerals.
Only when it contains lead: This statement is incorrect. Ores are not limited to containing only lead. Ores can contain various metallic minerals, not just lead.
When it contains at least one metallic mineral in sufficient amounts to be extracted profitably: This statement is correct. Ores are rocks that contain valuable metallic minerals in concentrations that make extraction economically feasible. These minerals can include gold, silver, copper, aluminum, zinc, and many others.
Only when it contains iron: This statement is incorrect. While iron ores are commonly known and widely used, ores are not limited to containing only iron. There are numerous other metallic minerals that can be extracted profitably from rocks.
When it contains fluorite or sulfur minerals in sufficient amounts to be extracted profitably: This statement is correct. Fluorite and sulfur are examples of non-metallic minerals that can be extracted profitably from rocks, and if a rock contains these minerals in sufficient quantities, it can be considered an ore.
learn more about from metallic mineral the given link:
https://brainly.com/question/89259
#SPJ11
What is the density?

Answers
Answer:
J. 20 g/cm³
Explanation:
Use the formula:
density = mass ÷ volume
mass = 100g
volume = 5cm³
Substitute (or plug in) the values into the formula:
density = 100 ÷ 5 = 20 g/cm³
20 points please don’t just put a answer I NEED HELP ASAP!!

Answers
determine+the+amount+of+potassium+chloride+(kcl)+present+in+a+500.0+ml+sport+drink+of+the+drinks+nutrition+label+shows+that+it+is+1.5%+kcl+by+mass.
Answers
There are approximately 7.5 grams of potassium chloride (KCl) present in the 500.0 mL sports drink.
To determine the amount of potassium chloride (KCl) present in the 500.0 mL sports drink, we need to calculate the mass of KCl based on the given percentage composition. Given:
Volume of sports drink = 500.0 mL
Percentage of KCl by mass = 1.5%
To find the mass of KCl, we can use the formula:
Mass of KCl = Percentage composition x Total mass of the solution
First, we convert the volume of the sports drink from millilitres to grams assuming the density of the solution is 1 g/mL:
Mass of the solution = Volume of the solution x Density
Mass of the solution = 500.0 mL x 1 g/mL
Mass of the solution = 500.0 g
Next, we calculate the mass of KCl using the percentage composition:
Mass of KCl = (Percentage of KCl / 100) x Mass of the solution
Mass of KCl = (1.5 / 100) x 500.0 g
Mass of KCl = 0.015 x 500.0 g
Mass of KCl = 7.5 g
To know more about potassium chloride (KCl), visit:
https://brainly.com/question/31236218
#SPJ11
A steam engine accepts heat during isothermal expansion at 250 °C and discards heat through isothermal compression at 25 °C. The magnitude of the entropy change during the isothermal compression step is 750 J/K. The efficiency of the steam engine is 65% of the maximum attainable theoretical efficiency. Calculate the total work produced by the engine. [12 marks]
Answers
The total work produced by the steam engine needs to be calculated and given that the steam engine accepts heat during isothermal expansion at 250 °C and discards heat through isothermal compression at 25 °C.
The magnitude of the entropy change during the isothermal compression step is 750 J/K. The efficiency of the steam engine is 65% of the maximum attainable theoretical efficiency. Given that the efficiency of the steam engine is 65% of the maximum attainable theoretical efficiency.
Hence, the actual efficiency will be:
η_actual = η_max × 0.65Where,
η_max is the maximum attainable theoretical efficiency.
η_actual = 0.65 × η_maxNow,
the efficiency of a Carnot engine (η_Carnot) can be given as:η_Carnot = 1 - (T_cold / T_hot)Here, T_hot and T_cold are the temperatures of the hot and cold reservoirs respectively.
To know more about isothermal visit:
https://brainly.com/question/29401722
#SPJ11
Work is a measure of the energy transfer that occurs when an object is moved against a force. Work is defined as the product of the force applied to an object and the displacement of the object in the direction of the force. Hence the total work produced by the engine is 47,175 J.
1. Converting the temperatures from degrees Celsius to Kelvin.
T1 = 250 °C + 273.15 K = 523.15 K
T2 = 25 °C + 273.15 K = 298.15 K
2. The maximum attainable theoretical efficiency.
ηmax = 1 - T2 / T1 = 1 - 298.15 / 523.15 = 42.9%
3. The efficiency of the steam engine.
η = 0.65 * ηmax = 0.65 * 42.9% = 27.7%
4. The entropy change during the isothermal expansion step.
dS = 750 J/K
5. The total work produced by the engine.
W = η * (T1 - T2) * dS = 0.277 * (523.15 - 298.15) * 750 J/K = 47,175 J
Therefore, the total work produced by the engine is 47,175 J.
To know more about Work:
https://brainly.com/question/31050706
#SPJ4
Why is it important for the mass to be measured before measuring the volume
Answers
Answer:
sorry if wrong
Explanation:
Answer:
mass of the solid is measured before measuring its volume to find the density
Answer:
So you can calculate the density.
Explanation:
Look at the following equation.
__Au2O3 → _Au + _02
In order to follow the law of conservation of mass, this equation must have which set of coefficients in order from left to right?
A. 2,4,3
B. 1,3,1
C. 2,1,3
D. 1,1,3
Answers
Answer:
A is correct
Explanation:
AU=2x2=4 Au=1x4=4
O=3x2=6 O=2x3=6
How do these momenta compare the ballon rocket?
Answers
in your own words describe the process of a physical change
Answers
Answer:
A physical change is when the form of an object changes, but the substance stays the same.
Example: Ice melting into water.
Look at the activity series and select which two of the following reactions would happen on their own. (Remember, if the lone element is more active than the metal in the compound, the lone element will react and replace the metal in the compound.) Lithium (Li)
Potassium (K)
Calcium (Ca)
Sodium (Na)
Aluminum (Al)
Zinc (Zn)
Iron (Fe)
Tin (Sn)
Lead (Pb)
(Hydrogen) (H)
Copper (Cu)
Silver (Ag)
Gold (Au)
A.
2Li + ZnBr2 ->2LiBr + Zn
B.
Al + 3LiCl ->AlCl3 + 3Li
C.
Sn + ZnSe ->SnSe + Zn
D.
3Ca + Al2O3 ->2Al + 3CaO
Answers
Answer:
2Li + ZnBr2 ->2LiBr + Zn
3Ca + Al2O3 ->2Al + 3CaO
Explanation:
Spontaneous reactions are reactions that can happen on their own. For a spontaneous reaction to occur, a metal that is higher in the activity series must displace a metal that is lower in the activity series from its solution and not vice versa.
If we look at the two reactions selected in the answer, lithium is above zinc in the activity series and calcium is above aluminum in the activity series hence the two reactions occur spontaneously.
The radius of an atom is dependent upon which 2 things? (Two answers)
A.) the number of electrons that it contains
B.) the number of neutrons that it contains
C.) the magnitude of attraction from its nucleus
D.) the distance between the electrons and its nucleus
Answers
Answer:
The correct options are;
C. The magnitude of attraction from its nucleus
D. The distance between the electrons and its nucleus
Explanation:
The atomic radius reduces, within a given period, as we move from left to right, the number of protons increases alongside the number of electrons and the while the quantum shell to which the extra electrons are added to is the same. Therefore, the radius of the atom is dependent on the magnitude of the attraction from the nucleus
Similarly, as we progress to the next period, with an extra quantum shell, the atomic radius is seen to increase.
Therefore, the atomic radius is determined by the distance between the electrons and its nucleus.
help me with bellwork

Answers
Answer:
1. PE
2. KE
3. PE
4. KE
5. KE
6. PE
7. KE
8. KE
9. PE
10. PE
Explanation:
Think about it this way, potential energy is stored energy while kinetic energy is active energy.
Why did we add laemmli buffer to the fish sample?
Answers
Answer:
To linearize the proteins
Explanation:
Laemmli buffer, which contains SDS, was added to fish samples to linearize the proteins by breaking up secondary and tertiary protein structure, ensuring that proteins will only move by size. Also, coats proteins to be electrically charged so it will migrate down the gel
radon-222 decays by a series of three α emissions and two β emissions. what is the final stable nuclide?
Answers
The final stable nuclide resulting from the decay of radon-222 is lead-206. Radon-222, also known as Rn-222, undergoes a process of radioactive decay.
During radioactive decay, Rn-222 emits three alpha particles (α) and two beta particles (β). An alpha particle consists of two protons and two neutrons, while a beta particle is either an electron (β-) or a positron (β+). As a result of this decay chain, the atomic number and mass number of the radon-222 nucleus change.
The decay process starts with the emission of an alpha particle, which reduces the atomic number of the nucleus by two units and the mass number by four units. This creates a new nucleus of polonium-218 (Po-218). The Po-218 nucleus further undergoes alpha decay, emitting another alpha particle and forming the stable nucleus of lead-214 (Pb-214).
The decay chain continues with the emission of a beta particle from Pb-214, converting a neutron into a proton and forming bismuth-214 (Bi-214). Bi-214 then undergoes another beta decay, emitting a second beta particle and producing the stable nucleus of polonium-214 (Po-214).
Finally, Po-214 decays through the emission of an alpha particle, resulting in the formation of lead-210 (Pb-210). Pb-210 subsequently undergoes further alpha decay, leading to the production of stable lead-206 (Pb-206). Therefore, the final stable nuclide resulting from the decay of radon-222 is lead-206.
Learn more about radioactive decay here:
https://brainly.com/question/1770619
#SPJ11
What is the H concentration in a solution with a pH of 1. 25? Round to the nearest hundredth. × 10n M n=.
Answers
The pH is the measure of hydrogen ion concentration in a solution. The hydrogen ion concentration at pH 1.25 is \(\rm\bold{ 5\;\times 10^{-2}\;M}\).
How to calculate hydrogen concentration?The pH is the negative logarithmic value of the hydrogen ion concentration in the sample.
The pH can be expressed as:
\(\rm pH=-log[H^+]\)
The hydrogen ion concentration can be given as:
\(\rm [H^+]=10^{-pH}\)
The given pH is 1.25 The hydrogen ion concentration is given as:
\(\rm [H^+]=10^{-1.25}\\H^+=0.05\;M\\H^+=5\;\times\;10^-^2\;M\)
The hydrogen ion concentration in the solution is \(\rm 5\;\times\;10^{-2}\;M\).
Learn more about pH, here:
https://brainly.com/question/491373
Answer: 5.62 x 10n M n=-2
(5.62) (-2)
Explanation:
What is viscosity?
A fancy word for energy
A liquid's flow
A type of plant
Answers
at a molecular level describe the differences between a hot object and a cold object
Answers
Answer:
.
Explanation:
The hot object are those which have high energy atoms that moves with a very fast energy whereas the cold object are those which have low energy atoms and moves with low speed.
A 3.00-g lead bullet is traveling at a speed of 240 m/s when it embeds in a wood post. If we assume that half of the resultant heat energy generated remains with the bullet, what is the increase in temperature of the embedded bullet? (specific heat of lead = 0.030 5 kcal/kg×°C, 1 kcal = 4 186 J)
Answers
The increase in temperature of the embedded bullet is approximately 112.6 °C.
We will find the increase in temperature of the embedded bullet using the given mass, speed, specific heat, and energy conversion factors.
- Mass of the bullet (m) = 3.00 g = 0.003 kg (convert grams to kilograms)
- Speed of the bullet (v) = 240 m/s
- Specific heat of lead (c) = 0.0305 kcal/kg×°C = 0.0305 × 4186 J/kg×°C (convert kcal to Joules)
- Half of the heat energy remains with the bullet
1. Calculate the initial kinetic energy (KE) of the bullet using the formula KE = 1/2 × m × v²
2. Divide the initial KE by 2 to find the heat energy absorbed by the bullet
3. Use the heat energy (Q), mass (m), and specific heat (c) to calculate the increase in temperature (ΔT) using the formula Q = m × c × ΔT
Now let's calculate:
1. KE = 1/2 × 0.003 kg × (240 m/s)² = 86.4 J
2. Heat energy absorbed by the bullet = 86.4 J / 2 = 43.2 J
3. 43.2 J = 0.003 kg × (0.0305 × 4186) J/kg×°C × ΔT
Solving for ΔT:
ΔT = 43.2 J / (0.003 kg × 127.673 J/kg×°C) ≈ 112.6 °C
You can learn more about the temperature at: brainly.com/question/11464844
#SPJ11
20 Points!!
Need it as soon as possible.
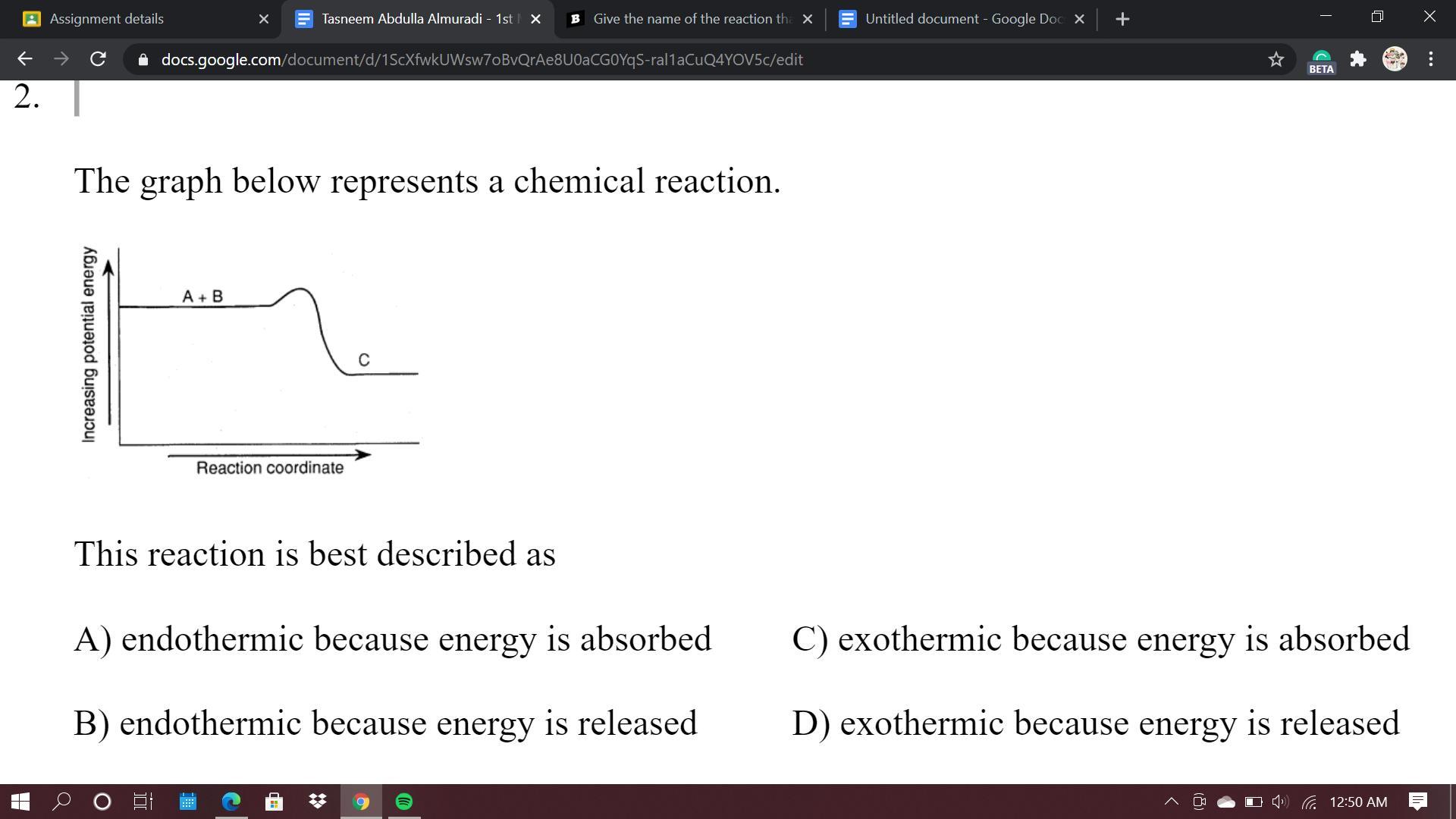
Answers
Answer:
D.
Explanation:
The reaction is losing potential energy, which means that the reaction is losing that energy as heat. Exothermic is the loss of energy. Therefore it will be D.
energy is being released in that.
Which type of reaction is NaCl + AgNO3 → NaNO3 + AgCl? synthesis decomposition oxidation replacement
Answers
Hope this helps! :)
The type of reaction represented by the equation is replacement reaction.
Definition of replacement reactionA replacement reaction also known as displacement reaction is a reaction in which the ions of the reactant exchange to produce a new product. For example
AB + CD —> AD + CB
In the above reaction, we can see that D replaces B in the product which shows that the reaction is a replacement reaction.
How to determine the type of reactionNaCl + AgNO₃ → NaNO₃ + AgCl
In the above reaction, we can see that Cl replaces NO₃ in the product. This simply indicates that the reaction is a replacement reaction.
Learn more about chemical reactions:
https://brainly.com/question/8625202
The monomer that is polymerized to make natural rubber is __________
Answers
Convert each into decimal form. a) 1.056 x 10-3 b) 0.560 x 102
Answers
Answer:
A. 7.56
b. 57.12
Explanation:
Hope that's right have a nice day :)