[2 pts] how do we prove kb |= d using a sat solver? (hint: the solution is simple and takes just one line.)
Answers
Use a SAT solver to see if kb d is unsatisfiable. In such case, kb |= d.
To prove kb |= d using a SAT solver, we need to check if the knowledge base kb together with the negation of the proposition d, i.e., (kb ∧ ¬d), is unsatisfiable. If the SAT solver finds that (kb ∧ ¬d) is unsatisfiable, then we know that d must be true given the knowledge base kb, since there is no possible assignment of truth values to the propositional variables that satisfies the knowledge base and makes d false. Using a SAT solver to check the satisfiability of (kb ∧ ¬d) is a standard method for performing logical entailment using automated reasoning. SAT solvers are efficient tools that can quickly determine the satisfiability of propositional formulas. If the formula (kb ∧ ¬d) is unsatisfiable, then the SAT solver will return a proof of unsatisfiability, which can be used to show that d logically follows from kb.
learn more about solver here:
https://brainly.com/question/29886190
#SPJ4
Related Questions
A 0. 150-mole quantity of CoCl2 is added to a liter of 1. 20 M NH3 solution. What is the concentration of Co2 ions at equilibrium? Assume the formation constant* of Co(NH3)62 is 5. 0 × 1031 M–6
Answers
The concentration of Co²⁺ ions at equilibrium is 0.150 M.
To determine the concentration of Co²⁺ ions at equilibrium, we need to consider the reaction between CoCl₂ and NH₃ to form Co(NH₃)₆²⁺.
The balanced equation for the reaction is:
CoCl₂ + 6NH₃ ⇌ Co(NH₃)₆²⁺ + 2Cl⁻
We can set up an ICE (Initial, Change, Equilibrium) table to solve for the concentration of Co²⁺ ions at equilibrium.
Initially, we have 0.150 moles of CoCl₂ and 1 liter of 1.20 M NH₃ solution.
Using the stoichiometry of the reaction, we can see that for every mole of CoCl₂, we form 1 mole of Co(NH₃)₆²⁺ ions. Therefore, the concentration of Co(NH₃)₆²⁺ ions at equilibrium is equal to the initial concentration of CoCl₂.
Therefore, the concentration of Co²⁺ ions at equilibrium is 0.150 M.
learn more about ions here
https://brainly.com/question/30663970
#SPJ11
Which transfers thermal energy in the same way the Suns energy is transferred to Earth?
A. The boiling water
B. The burner flame
C. The hot candle
D. The rising steam
Answers
Answer: I think this one is the boiling water
sorry if wrong
Explanation:
PLEASEE HELP IM DESPERATE

Answers
You'll have 20.5 g of hydrochloric acid (HCl) left, which is the answer to your query.
The least amount of hydrochloric acid that can be produced?Data
HCl = 31.4 g
NaOH = 12 g
insufficient HCl =?
equilibrium in a chemical reaction
NaCl and H2O are produced from HCl and NaOH.
HCl's molar mass is equal to 1 + 35.5 = 36.5 g.
NaOH's molar mass is 40 g (23 + 16 + 1)
Figure out the limiting reactant.
Theoretical yield: 36.5/40 = 0.9125 for HCl/NaOH.
Research yield: HCl/NaOH = 31.4/12 = 2.62
Conclusion
Because of the increasing experimental yield, NaOH is the limiting reactant.
The excess reactant's mass should be determined.
35. 5 g of HCl ———————— NaOH weight 40 g
x ———————— NaOH in 12 g
x = (12 x 36.5) / 40
x = 438 / 40
x = 10.95 grams of HCl
Surplus HCl = 31.4 - 10.95
= 20.5 g
You'll have 20.5 g of HCl left, which is the answer to your query.
The complete question is,
To create aqueous sodium chloride and liquid water, aqueous hydrochloric acid must be combined with solid sodium hydroxide. Consider combining 12.g of sodium hydroxide with 31.4g of hydrochloric acid. Determine the smallest amount of hydrochloric acid that could possibly remain after the reaction. The quantity of significant digits in your response must be accurate.
To learn more about reactant's mass refer to:
https://brainly.com/question/19958395
#SPJ1
whoever answers first will get brainliest! this is times so pls help!
You are titrating a 125 mL sample of KOH solution; the pH meter reads 7.00 after the addition of 84.7 mL of 0.750 M HNO3.
What is the concentration of the KOH solution?
A. 0.125 M KOH
B.0.750 M KOH
C.0.508 M KOH
D. 0.0635 M KOH
thanks so much!
Answers
1.
what can noble gases be used for?
2.
what is a vertical column called in the periodic table
3.
what is the horizontal column in the periodic table
Answers
2. Groups
3. Rows
Answer:
1. They are mostly used to protect materials and prevent chemical reactions.
2.These are Groups
3. I guess it is Rows
What length should a bagpipe pi ends and is being played at room temperature. pe have to produce a fundamental frequency of 131 Hz ? Assume the pipe is open at both
Answers
The length of the bagpipe pipe should be approximately 4.3 feet long in order to produce a fundamental frequency of 131 Hz when played at room temperature.
The fundamental frequency of a pipe is determined by its length and the speed of sound in the medium it is traveling through. In this case, the pipe is open at both ends, which means it is a type of pipe known as an open-open pipe. The formula for calculating the fundamental frequency of an open-open pipe is:
f = (n * c) / (2 * L)
Where f is the frequency, n is the harmonic (in this case, the fundamental frequency is the first harmonic), c is the speed of sound (which is approximately 343 meters per second at room temperature), and L is the length of the pipe.
To solve for L, we can rearrange the formula:
L = (n * c) / (2 * f)
Plugging in the values we have (n = 1, c = 343 m/s, and f = 131 Hz), we get:
L = (1 * 343 m/s) / (2 * 131 Hz)
L = 1.31 meters, or approximately 4.3 feet.
To learn more about fundamental frequency visit:
brainly.com/question/29264927
#SPJ11
Plant and Animal Cells
С
B
F
1 20 points
Which letter represents the brain of the cell?
о B
H
H
Why
E
D
G

Answers
Answer:
Nucleus is known as the brain of the cell.
correct option is B letter
i need help please........

Answers
Answer:
cd
Explanation:
image is formed due to reflected light rays and it is enlarged because image distance/ object distance = height of image/height of object
v>u so image is enlarged
Thanks for you help:)

Answers
Answer:
they spread out waiting until each orbital has one electron before doubling up (the last bullet point)
Explanation:
This is because electrons are negatively charged so if they doubled up together in the begging, they would repel each other.
Identify which properties are common to each of the following chemical families
(a) alkali metals
(b) alkaline earth metals
(c) halogens
(d) noble gases
Answers
The noble gases have a full outer shell of valence electrons, making them stable and unreactive. They are colorless, odorless gases at room temperature and have very low boiling points. Their lack of reactivity makes them useful in a variety of applications, including lighting and welding.
The properties that are common to each of the following chemical families include:
(a) Alkali metals The alkali metals have a single valence electron in their outermost shell, which is easily lost to form an ion with a charge of +1. They are the most reactive metals, reacting with water and air to produce hydrogen gas and an oxide layer, respectively. They are silvery-white and have a soft texture.
(b) Alkaline earth metals The alkaline earth metals have two valence electrons in their outermost shell, which they readily lose to form ions with a charge of +2. They are less reactive than the alkali metals, but they still react with oxygen to form an oxide layer on their surface. They are also silvery-white in color and have a harder texture than the alkali metals.
(c) Halogens The halogens have seven valence electrons in their outermost shell, making them highly reactive nonmetals. They readily form ions with a charge of -1 by gaining an electron. They are diatomic molecules at room temperature and can be found in a variety of colors and states of matter.
(d) Noble gases The noble gases have a full outer shell of valence electrons, making them stable and unreactive. They are colorless, odorless gases at room temperature and have very low boiling points. Their lack of reactivity makes them useful in a variety of applications, including lighting and welding. These properties are common to each of the following chemical families.
To know more about noble gases visit:-
https://brainly.com/question/19024000
#SPJ11
of the planet's surface is coverod with the liquid. (Type an exact answer, using at as neveded.)
Answers
The units for both the area covered with liquid and the total surface area of the planet are the same before performing.
To determine the percentage of the planet's surface covered with liquid, you need to follow these steps:
Step 1: Determine the total surface area of the planet.
Find the radius (or diameter) of the planet. Let's say the radius is given as "r" units.
Calculate the surface area of a sphere using the formula: A = 4πr². This gives you the total surface area of the planet.
Step 2: Determine the surface area covered with liquid.
Estimate or obtain the area covered by liquid on the planet. Let's say this area is given as "A_liquid" units².
Step 3: Calculate the percentage of the planet's surface covered with liquid.
Divide the area covered with liquid (A_liquid) by the total surface area of the planet.
Multiply the result by 100 to get the percentage.
Mathematically, the calculation can be represented as:
Percentage = (A_liquid / Total surface area) x 100
Ensure that the units for both the area covered with liquid and the total surface area of the planet are the same before performing the calculation.
Remember to substitute the given values into the formula to obtain the final percentage of the planet's surface covered with liquid.
Learn more about surface area from the given link
https://brainly.com/question/951562
#SPJ11
At which position on the diagram would an apple have the greatest potential energy?
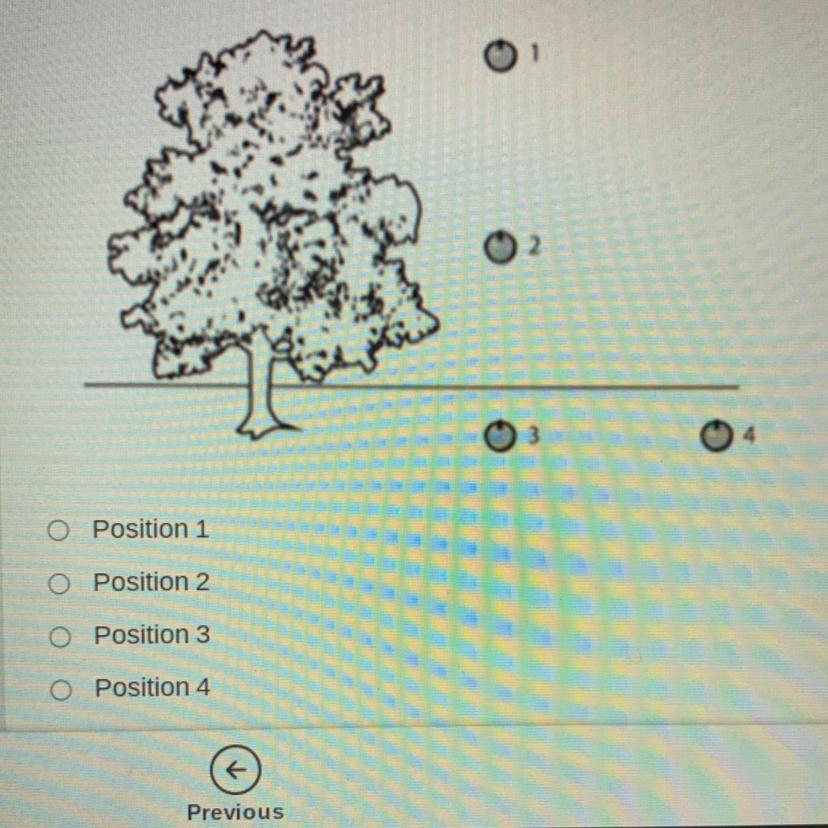
Answers
Answer:
Position 1 because it is the highest from the ground.
Hope this helps!
A certain hydrocarbon has a chemical composition of 79.85% of carbon and 20.15% of hydrogen. The molar mass is 60.16 g/mol. What is the molecular formula for this compound
Answers
The molecular formula for the compound is \(C_{4} H_{8}\) .
Given:% of C = 79.85%
% of H = 20.15%
Let the mass of the compound be 100g, then
Mass of C = 70.85 g
Mass of H = 20.15 g
Molar mass = 60.15 g/mol
Moles of Carbon = \(\frac{79.85}{12.01 g/mol}\)
= \(6.64 mol\)
Moles of Hydrogen = \(\frac{20.15}{1.00794 g/mol}\)
= \(19.99 mol\)
And we divide both sides by the lower quantity;
\(C_{\frac{6.64 mol}{6.64 mol} } H_{\frac{19.99 mol}{6.64 mol} }\) ≅ CH₂
And \((empirical formula)_{n} = molecular formula\)
60.15 g/mol = n × ( 12.01 + 2 × 1.00794)
n = 4
Therefore, the molecular formula for the compound is \(C_{4} H_{8}\) .
Learn more about molecular formula here:
brainly.com/question/15960587
#SPJ4
when the reaction cl2 (aq) -> cl- clo3- is balanced in aqueous solution, wha tis the coefficient of h2o
Answers
The balanced equation for the reaction Cl2 (aq) -> Cl- + ClO3- in aqueous solution is:
3 Cl2 (aq) + 6 H2O (l) -> 5 Cl- (aq) + ClO3- (aq) + 6 H+ (aq)
When a chemical substance is said to be "balanced in aqueous solution," it means that the substance is completely dissolved in water and has undergone a chemical reaction where the number of atoms of each element present in the reactants is equal to the number of atoms of each element present in the products. the coefficient of H2O in the balanced equation is 6. This means that 6 molecules of water are required for every 3 molecules of Cl2 that react to produce 1 molecule of ClO3-.
The coefficients represent the stoichiometric ratios between the reactants and products, which can provide important information about the quantities of substances involved in the reaction.
To know more about reaction visit :-
https://brainly.com/question/14158846
#SPJ11
What are these means in Periodic Table;
Group
Period
Block
Answers
2. Period: Periods show the number of energy levels each element has and are shown in rows on the periodic table (up and down).
3. Blocks are show the amount of orbitals each element has. There is an s block, d block, p block, and f block.
chloride per milliliter (MW of CaCl2 = 147) [Round to the nearest whole number 5. What weight of magnesium chloride (MgCl2, formula weight = 95.3) is required to prepare 200 ml solution that is 5.0 mi
Answers
The weight of magnesium chloride required to prepare the 200 ml solution that is 5.0 M is approximately 48 grams.
To calculate the weight of magnesium chloride (\(MgCl_{2}\)) required to prepare a 200 ml solution that is 5.0 M, we need to use the formula: Weight (in grams) = Volume (in liters) × Concentration (in moles/liter) × Molecular Weight (in grams/mole)
First, we convert the volume from milliliters to liters by dividing it by 1000: Volume = 200 ml ÷ 1000 = 0.2 L. Next, we multiply the volume, concentration, and molecular weight: Weight = 0.2 L × 5.0 mol/L × 95.3 g/mol = 47.65 grams
Rounding to the nearest whole number, the weight of magnesium chloride required to prepare the 200 ml solution that is 5.0 M is approximately 48 grams.
This calculation ensures that the desired concentration is achieved by accurately measuring the appropriate amount of magnesium chloride, taking into account its molecular weight and the desired volume of the solution.
To know more about magnesium chloride, refer here:
https://brainly.com/question/30671024#
#SPJ11
16. If you have 12.04 * 1023 molecules of carbon, how many moles of carbon do you
have?
Answers
Answer:
2 molesExplanation:
To find the number of moles in a substance given it's number of entities we use the formula
\(n = \frac{N}{L} \\\)
where n is the number of moles
N is the number of entities
L is the Avogadro's constant which is
6.02 × 10²³ entities
From the question we have
\(n = \frac{12.04 \times {10}^{23} }{6.02 \times {10}^{23} } = \frac{12.04}{6.02} \\ \)
We have the final answer as
2 molesHope you this helps you
Does pure water conduct electricity? if not, what can we do to make it conducting?
Answers
Explanation:
Pure water does not conduct electricity. This is because pure water do not contain any salts. Pure water can conduct electricity when common salt is added to it, as salt solution is conducting in nature.
what happens to the particles of a solid as it becomes a liquid
Answers
Answer:
melting
Explanation:
Answer:
When a solid is heated the particles gain energy and start to vibrate faster and faster. ... Further heating provides more energy until the particles start to break free of the structure. Although the particles are still loosely connected they are able to move around. At this point the solid is melting to form a liquid.
Explanation:
When the bromine atom becomes an anion, it ______ in size.
Group of answer choices
A. increase
B. decrease
C. remains the same
When the strontium atom becomes a cation, it ______ in size.
A. increase
B. decrease
C. remains the same
Answers
Explanation:
Anions and cations have different atomic sizes than the neutral atoms.
When the bromine atom becomes an anion, it ______ in size.
Anions involves gaining extra electrons. This basically leads to increased repulsion between the electrons and thereby increasing the size. Anions are always larger than the neutral atoms.
This means the answer is A. Increase
When the strontium atom becomes a cation, it ______ in size.
Cations involves losing valence electrons. This basically leads to a decrease in electron shells and electron repulsion thereby reducing the size. Cations are always larger than the neutral atoms.
This means the answer is B. Decrease
Find the mass of a block of ice that has the dimensions of 5cm height, 6cm long, and 10cm in width and a density of 30 grams / c m~3
Answers
The mass of a block of ice that has the dimensions of 5cm height, 6cm long, and 10cm in width and a density of 30 grams / c m³ is 9000 g.
given that :
density = 30 g/ cm³
height of block = 5cm
length of block = 6cm
width of block = 10 cm
volume of the block is calculated by the following formula :
volume = length × width × height
volume = 6 cm × 10 cm × 5 cm
volume = 300 cm³
now, the density of the block is given by :
Density = mass / volume
mass = Density × volume
mass = 30 g/ cm³ × 300 cm³
mass = 9000 g
Thus, The mass of a block of ice that has the dimensions of 5cm height, 6cm long, and 10cm in width and a density of 30 grams / c m³ is 9000 g.
To learn more about Density here
https://brainly.com/question/10786454
#SPJ1
A chemist is identifying the elements present in a sample of a sea water. What characteristics of an element's
atoms always determines the element's identity?
A.
B
C.
D
The number of protons
The number of neutrons
The location of valence electrons
The number of valence electrons
Answers
Answer:
The number of valence electrons
What is deforestation?
A
the process of finding medicine ingredients in the rainforest
B
the planting of new trees on empty land
с
the process by which trees soak up carbon dioxide
D
the cutting down of existing forests
Answers
Answer:
D.
Explanation:
The action of clearing a wide area of trees.
Deforestation is the action of clearing a wide area of trees.
Read the water level with the correct number of significant figures.
1) 5 mL
2) 5.3 mL
3) 5.32 mL
4) 5.320 mL
5) 5.3200 mL

Answers
Answer:
the answereis is 5 ml
Explanation:
hope thhis helped.
Think about a wave model with a rope. How do you think the size of the wave going through the rope is affected by how far you moved your arm up and down or how far you moved your arm back and forth?
A.
The farther I move my arm, the larger the size of the wave in the rope.
B.
The less I move my arm, the larger the size of the wave in the rope.
C.
How far I move my arm has no effect on the size of the wave in the rope.
Answers
Given that the disturbance travels along the length of the rope, the farther I move my arm, the larger the size of the wave in the rope.
What is a wave?A wave is a disturbance along a medium which transfers energy. We know that a wave could be classified based on the nature of the propagation of the wave as the transverse or the longitudinal waves.
The wave that is created along a string is a longitudinal wave. The direction of the wave motion is the same as that of the disturbance. It is very easy to create a wave on a string when the sting is moved vigorously and the wave is found to travel quickly across the length of the rope is we can see from the image that I have attached to make the concept and the context of the answer a bit more clearer to the student in this case.
Learn more about longitudinal wave:https://brainly.com/question/8497711
#SPJ1

What is the volume of a bar of soap that has a density of 2.5 g/cm3 and a mass of 100 g?
O4 cm3
O 0.4 cm3
O 400 cm3
O 40 cm3
Answers
The volume of a bar of soap that has a density of 2.5 g/cm3 and a mass of 100 g is 40 cm ³. Therefore, option D is correct.
What is density ?The mass of a substance per unit of volume is its density. A mechanically accepted measure is density. Density is most frequently represented by the symbol, however the Latin letter D may also be used.
The mass of a solid substance's unit volume. d = M/V, where d is density, M is mass, and V is volume, is the formula for density. Grams per cubic centimeter are a typical unit of measurement for density.
Density = m / V
volume = mass / density
= 100 / 2.5
= 40 cm³
Thus, option D is correct.
To learn more about the density, follow the link;
https://brainly.com/question/6107689
#SPJ1`
the first ionization energy of selenium is 941 kj/mol. calculate the maximum wavelength of a photon that can ionize se.
Answers
The greatest wavelength at which a photon may get ionized is 2.294 107 nm. When an atom or molecule acquires or eliminates an energy, the activity of ionization creates ions.
The ionization energy rule is what?
Upon that annual chart, that the very first oxidation number typically climbs towards left to right for each period. The higher nuclear charge results in a closer bond between the outermost electron and the nucleus.
How does ionization work?
Any process by which electrically neutral atoms or molecules are changed into electrically charged atoms or molecules (ions) by the gain or loss of electrons is referred to as ionization in chemistry and physics.
Briefing:
ionization energy= 941kj/mol
ionization energy= 941×1/6.022×10²³
=15.62×10⁻²²kj/atom
E=h∨
v=15.62×10⁻²²×10³/6.626×10⁻³⁴
=1.3075×10¹⁰s⁻¹
λν=c
λ=3×10⁸/1.3075×10¹⁰
=2.294×10⁻²m
To know more about ionization energy visit:
https://brainly.com/question/28385102
#SPJ4
An atom contains 22 protons and 26 neutrons. What is its mass number?
Answers
Answer:
47.867
Explanation: It's Titanium because the atomic number is the number of protons.
A quantity of monatomic ideal gas expands adiabatically from a volume of 2.0 liters to 6.0 liters. if the initial pressure is p0, what is the final pressure?
Answers
Answer:
1/3p0
Explanation:
The combined gas law:
P1V1/T1 = P2V2/T2, where P, V and T are Pressure, Volume, and Temperature. Temperature must always be in Kelvin. The subscriopts 1 and 2 are for initial (1) and final (2) conditions.
In this case, temperature is constant (adiabatically). V1 = 2.0L and V2 = 6.0L. I'll assume P1 = p0.
Rearrange the combined gas law to solve for final pressure, P2:
P1V1/T1 = P2V2/T2
P2 = P1*(V1/V2)*(T2/T1) [Note how I've arranged the volume and temoperature terms - as ratios. This helps us understand what the impact of raising or lowering one on the variables will do to the system].
No enter the data:
P2 = P1*(V1/V2)*(T2/T1): [Since T2 = T1, the (T2/T1) terms cancels to 1.]
P2 = p0*(2.0L/6.0L)*(1)
P2 = (1/3)p0
The final pressure is 1/3 the initial pressure.
As you move down the periodic table atoms get bigger. This is because.