1 You place a 28.95-g piece of gold in a 10-ml graduated cylinder. The level of the water rises 1.50 mL. What is the density of gold?
Answers
Answer:
What mass of silver will raise the level of the water in the graduated cylinder 1.50 mL? (Ans) 28.95 g / 1.50 mL = 19.3 g/mL (The gold's density)
19.3 gm/ml³ is the density of gold .
What do you mean by the density of the substance?Density, mass of a unit volume of a material substance. Density of a substance tells how close the particles of a substance are packed.
The formula for density is D = M/V, where d is density, M is mass, and V is volume.
Density is commonly expressed in units of grams per cubic centimeter.
The SI unit of density is kilogram per cubic meter (kg/m3).
It is also represented in the c g s unit of grams per cubic centimeter (g/cm3).
To calculate density of gold (here):
Given,
Mass of gold=28.95 gm
Volume of water=1.50 mL
Density=?
Density =Mass/Volume
Density=28.95/1.50
Density=19.3 gm/ml³
Hence, 19.3 gm/ml³ is the density of gold .
Learn more about density ,here:
https://brainly.com/question/15164682
#SPJ5
Related Questions
Methane, ch4, is the gas commonly found in labs to fuel bunsen burners. A) how many moles of methane are there in a 7. 21 gram sample?
Answers
Using the molar mass of methane, there are 0.45 mole of methane in a 7.21 gram sample.
Molar mass is defined as the mass per unit mole of a substance.
MM = m/n
where MM = molar mass
m = mass
n = number of moles
The molar mass of methane is the sum of the molar masses of each element.
C : 12.011
H : 1.00784
CH4 : 12.011 + 4(1.00784) = 16.04236 g/mol
If a sample of methane has a mass of 7.21 grams, using the formula for the molar mass, solve for the number of moles.
MM = m/n
16.04236 g/mol = 7.21 g/n
n = 7.21 g/16.04236 g/mol
n = 0.4494351205 mol
n = 0.45 mol
Learn more about molar mass here: https://brainly.com/question/15476873
#SPJ4
what does matter have besides mass.
Answers
Answer:
so like Each of the five states of matter collectively made up all the "stuff" that's in the universe- — everything that takes up space and has mass is matter,and All matter is made up of atoms and in turn made up of protons and neutrons and electrons.
Explanation: hope this helps
Calculate the mass of one mole of these substances.
a. NH4Cl
b. NH4NO3
c. AlCl3
Helppp plss :)
Answers
Answer:
a. 53.5 g/mol
b. 80.06 g/mol
c. 133.33 g/mol
General Formulas and Concepts:
Chemistry - Atomic Structure
Reading a Periodic TableMolar Mass - 1 mol per x grams substanceExplanation:
Step 1: Define
a. NH₄Cl
b. NH₄NO₃
c. AlCl₃
Step 2: Find masses
Molar Mass of N - 14.01 g/mol
Molar Mass of H - 1.01 g/mol
Molar Mass of O - 16.00 g/mol
Molar Mass of Al - 26.98 g/mol
Molar Mass of Cl - 35.45 g/mol
Step 3: Calculate compound masses
Molar Mass of NH₄Cl - 14.01 g/mol + 4(1.01 g/mol) + 35.45 g/mol = 53.5 g/mol
Molar Mass of NH₄NO₃ - 2(14.01 g/mol) + 4(1.01 g/mol) + 3(16.00 g/mol) = 80.06 g/mol
Molar Mass of AlCl₃ - 26.98 g/mol + 3(35.45 g/mol) = 133.33 g/mol
****************************************************************
Answers
******************* ijustwantthepoints *************************
what is the name that corresponds to the definitions of the following terms: : chemosensory organs that detect dissolved chemicals
Answers
Chemoreceptors are an important component of many physiological processes and play a key role in many behaviors, including feeding, mating, and avoidance of predators. They are also involved in the immune response and other physiological processes. In conclusion, chemoreceptors are chemosensory organs that detect dissolved chemicals.
The name that corresponds to the definition of "chemosensory organs that detect dissolved chemicals" is chemoreceptors. They are sensory receptors that detect specific chemical stimuli in the environment and transmit information to the brain for further processing.Chemoreceptors are found in a variety of organisms, including insects, crustaceans, and mammals. They are responsible for detecting chemicals that are dissolved in air, water, or other liquids. For example, in humans, the sense of taste is mediated by chemoreceptors located on the tongue that detect the presence of different chemicals in food. In contrast, chemoreceptors in the nose are responsible for detecting the presence of different odors in the air.Chemoreceptors are an important component of many physiological processes and play a key role in many behaviors, including feeding, mating, and avoidance of predators. They are also involved in the immune response and other physiological processes. In conclusion, chemoreceptors are chemosensory organs that detect dissolved chemicals.
To know more about Chemoreceptors visit:
https://brainly.com/question/13257699
#SPJ11
Clear selection
An atom is composed of electrons, protons, and neutrons. What is the
electric charge of a neutron?
O points
+1
-1
It depends
Answers
Answer:
hbjvcbhjkcvbfhg
Explanation:
relative to the inside of a cell, what is meant when the surrounding solution is describes as being:
Answers
The inside of a cell, what is meant when the surrounding solution is is called hypertonic solution.
What is hypertonic solution?
When a cell is immersed in a hypertonic solution, there is a net flow of water out of the cell, resulting in volume loss. A solution is hypertonic to a cell if its solute concentration exceeds that of the cell, and the solutes are unable to penetrate the membrane.
A hypertonic solution is a solution that has a higher concentration of solutes on the exterior of a cell than on the inside of a cell.
A hypertonic solution has a higher osmotic pressure than another solution. In other terms, a hypertonic solution has a higher concentration or quantity of solute particles outside a membrane than inside it.
The traditional example used to describe tonicity is red blood cells. When the concentration of salts (ions) inside the blood cell is the same as the concentration outside, the solution is isotonic with respect to the cells, and they resume their usual form and size.
If there are less solutes outside the cell than inside it, as if red blood cells were placed in fresh water, the solution (water) is hypotonic in comparison to the interior of the red blood cells. As water rushes into the cell to try to equalise the concentrations of the interior and outer solutions, the cells enlarge and may explode.
Learn more about hypertonic solution from given link
https://brainly.com/question/28644153
#SPJ4
.Which of the following are characteristics of a gas? It moves slowly. It has a fixed volume. It can be compressed. It has a fixed shape.
Answers
Answer:
It has a fixed volume it can also be compressed
Explanation:
1: A gases molecules don't move slow because they are not solid and are not compacted.
Answer:
It can be compressed
Explanation:
The Properties of Gases. Gases have three characteristic properties: (1) they are easy to compress, (2) they expand to fill their containers, and (3) they occupy far more space than the liquids or solids from which they form.
Help pretty please jdjsmsjsj

Answers
Answer:
less metallic
Explanation:
Answer:
D; Decrease in atomic mass.
Explanation:
I looked at the periodic table.
The specific shape that an electron moves in inside a sub-level_____
Answers
Answer:
The specific shape that an electron moves in inside a sub-level shell.
explain how rutherford’s model of the atom differed from earlier models of the atom dalton
Answers
Rutherford's model of the atom differed significantly from earlier models, such as Dalton's atomic theory. Here are some of the key differences:
Structure of the atom: Dalton's atomic theory proposed that atoms were indivisible, solid spheres. In contrast, Rutherford's model suggested that atoms had a complex structure consisting of a dense, positively charged nucleus at the center, surrounded by negatively charged electrons orbiting around it.
Charge of the nucleus: Dalton's model had no concept of a nucleus, so it did not address the charge of the center of the atom. Rutherford's model, on the other hand, proposed that the nucleus had a positive charge, which was responsible for the electrostatic attraction that kept the negatively charged electrons in orbit.
Size of the atom: Dalton's model did not propose a specific size for atoms. Rutherford's model, however, suggested that atoms were much smaller than previously believed, with most of the mass concentrated in the nucleus, which was thousands of times smaller than the overall size of the atom.
Experimental evidence: Rutherford's model was based on experimental evidence from his famous gold foil experiment, in which he shot alpha particles at a thin sheet of gold foil and observed how they were deflected. This provided direct evidence for the existence of a small, positively charged nucleus at the center of the atom. In contrast, Dalton's model was based more on theoretical considerations and did not rely as heavily on experimental evidence.
Rutherford's model of the atom differed from Dalton's model in several key ways, including its proposed structure, charge, size, and the experimental evidence used to support it. Rutherford's model represented a major advance in our understanding of the atom and provided a framework for later developments in atomic theory.
Learn more about atomic model here: brainly.com/question/28825274
#SPJ4
An induced dipole-dipole interaction has been proposed between aromatic amino acid residues present in the cholinergic binding site and acetylcholine. What functional group of acetylcholine is involved?
a) the acyl methyl group.
b) the ester.
c) the ethylene bridge.
d) the quaternary ammonium head group.
Answers
Answer:
hi-
Explanation:
interaction with acetylcholine?
Aspartic acid 311
10) What amino acid in the binding site of the cholinergic receptor is involved in an ionic interaction with acetylcholine? Feedback: Aspartic acid 311 is ionised and is present in the binding site as a negatively charged aspartate ion.
HELP ME PLEASEE!!!! BRAINLIEST FOR CORRECT ANSWER NO COPY AND PASTE. CLICK ON THE IMAGE

Answers
Search Results
Featured snippet from the web
The law of conservation of energy states that energy can neither be created nor destroyed - only converted from one form of energy to another. This means that a system always has the same amount of energy, unless it's added from the outside. ... The only way to use energy is to transform energy from one form to another.
Conversion of energy describes that energy cannot be created or destroyed, when work is done, energy is changed from one form to another.
An example of conversion of energy is when a book is on a shelf it has gravitational potential energy, if it falls of the shelf it will have kinetic energy.Though due to the process through which energy transfers take place not being 100% efficient, energy is sometimes lost to the surrounding and energy gets more spread out, air resistance and friction.
Some types of energy:
GravitationalKinetic energyStrain energyNuclear energyInternal EnergySound energyElectric energy.Calculate the mass of ammonium sulfide (NH4)2S in 3.00 L of a 0.0200 M solution.
Answers
The mass of (NH4) 2S in the solution is : Mass = 0.0600 mol × 60.08 g/mol = 3.60 g.
The given molarity and volume of the solution can be used to calculate the number of moles of ammonium sulfide (NH4)2S.Then, the number of moles can be converted to mass using the molar mass of (NH4)2S.Mass of ammonium sulfide (NH4)2S in 3.00 L of a 0.0200 M solution is given by : Mass = moles × molar mass.The number of moles of (NH4)2S can be found using the equation:Molarity = Number of moles / Volume.Rearranging this equation, we get:Number of moles = Molarity × Volume Number of moles of (NH4)2S = 0.0200 M × 3.00 L.Number of moles of (NH4)2S = 0.0600 mol.The molar mass of (NH4)2S can be calculated by summing the molar masses of ammonium (NH4) and sulfide (S) ions.Molar mass of (NH4)2S = (2 × Molar mass of NH4) + Molar mass of S= (2 × 14.01 g/mol) + 32.06 g/mol= 60.08 g/mol.
For more question on mass
https://brainly.com/question/1838164
#SPJ8
phenolphthalein indicator turns blue in basic solutions.true false
Answers
The statement that phenolphthalein indicator turns blue in basic solutions is false. Phenolphthalein is an indicator that turns pink in basic solutions. In acidic solutions, it remains colorless.
The explanation for this color change is the different ionized forms of phenolphthalein reacting with acidic or basic solutions, causing the visible color shift. Phenolphthalein is an indicator that changes color depending on the pH of the solution it is added to. In basic solutions, phenolphthalein turns pink. Phenolphthalein is added to a solution. The pH of the solution determines the color change of the indicator. In basic solutions, the phenolphthalein indicator turns pink. This is because the basic solution has a pH greater than 7, which causes the phenolphthalein to change to its pink form.
Learn more about phenolphthalein here:
https://brainly.com/question/15211751
#SPJ11
HELP ILL MATK YOU AS BRAINLEST
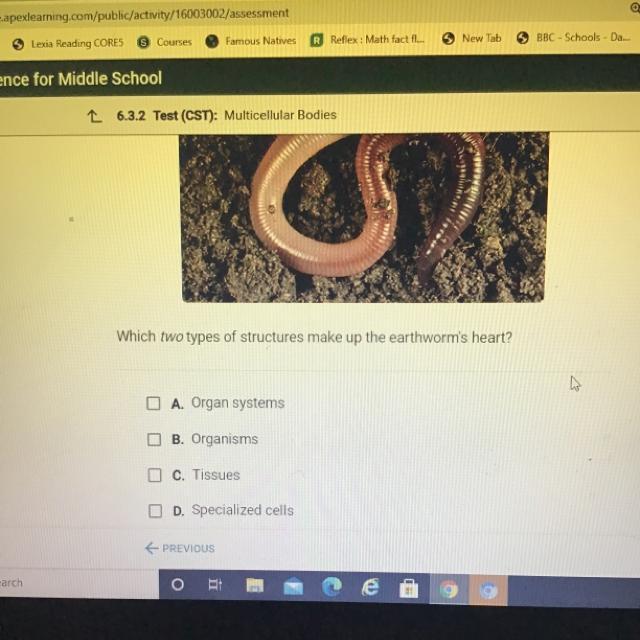
Answers
Answer:
Tissues And Organ Systems
Explanation:
What mineral is used in paint and roofing, is nonmetallic, has cleavage, and a hardness of 2?
Answers
Answer:
Muscovite is the mineral
Consider the substances quartz (SiOs), gold (Au), and nitrogen gas (N2). If possible, use the terms
molecule and compound to describe each substance. If one or more of the substances cannot be
described by the terms molecule and compound, explain why not.
Answers
Answer:
Silicon Dioxide is a compound usually referred to as Quartz, it's a crystalline sort of material that is commonly found and used for equipment and furniture (like tables for example). Their molecular geometry is of tetrahedral shape (how their bonds look like and such)
Gold is an element that is a transitional metal that is quite malleable (able to hammer it to sheets) and is ductile (can be made into wires). As you probably have guessed, gold is used a lot for jewelry due to its lustrous appearance, and the fact that it can't corrode as well as its rarity. Due to it not being able to corrode, it's pretty useful in circuitry as it requires less management unlike elements with higher electrical conductivity like copper or silver. However gold is pretty costly so it's not often relied on for technology and mostly just used for show instead.
N2 or nitrogen gas, may seem like a compound for people new to chemistry but is actually just a diatomic molecule, meaning they are composed of only 2 atoms. So elements that are diatomic are commonly found with only 2 atoms (Oxygen gas for example, or Hydrogen gas) Nitrogen is part of the "magnificent seven" (the elements which are diatomic". Nitrogen is essential for a lot of living things (aside from us), and since they are the most abundant material in our air (78% of air is Nitrogen), there's plenty of life growing.
Compound and elements are differ from each other. Silicon dioxide ( SiO₂ ) is compound , gold ( Au ) is element and nitrogen gas ( N₂ ) is compound.
What is difference between molecule and elements ?Elements are always made-up of only one type of atom. Molecule is made up of more than one type of atom. Silicon dioxide is a compound, which is crystalline in nature. Their molecular geometry is of tetrahedral shape.
Gold is an element that is a transitional metal that is quite malleable and is ductile. Gold ( Au ) is used a lot for jewelry. Nitrogen gas is compound . It is diatomic in nature, So elements that are diatomic are commonly found with only 2 atoms.
Hence, silicon dioxide is compound, gold is element, and nitrogen gas is also compound.
To learn more about molecules and elements follow the link below;
https://brainly.com/question/15304687
#SPJ2
Where does the heat that is needed to warm the surface of the ocean come from? A. the Sun B. the Earth's interior C. animals D. the Earth's surface
Answers
Answer:
A. The Sun
Explanation:
Answer:
A
Explanation:
Most of our heat source on Earth comes from the Sun.
What energy transformations took place in transforming chemical energy into kinetic energy? How many energy transformations can you list in this process?
Answers
Answer:
Energy transformations are processes that convert energy from one type (e.g., kinetic, gravitational potential, chemical energy) into another. Any type of energy use must involve some sort of energy transformation
Explanation:
Hii army hope it will help you
Energy transformations are procedures that transform energy from one type to another (e.g., kinetic energy, gravitational potential energy, chemical energy). Energy transformation is required for any type of energy use.
What is energy transformation?The process of converting energy from one form to another is referred to as energy transformation. Energy is a quantity in physics that provides the capacity to perform work or move (e.g., lifting an object) or provides heat.
Energy can be transferred in two ways: by doing work and by heat transfer.
Energy can be transferred from one location to another or from one object to another in three ways: through the movement of objects, the movement of waves, and the movement of heat.
Energy transformation, also known as energy conversion, is the process of transforming energy from one form to another. The exchange of thermal energy via conduction, convection, and radiation is known as heat transfer.
Electrical energy can be created by converting chemical energy. Heat energy can be created by converting thermal energy. Mechanical energy can be converted to electrical energy, potential energy, and other forms of energy.
Thus, this way, transformations took place in transforming chemical energy into kinetic energy.
For more details regarding energy transformation, visit:
https://brainly.com/question/29102331
#SPJ2
which of the following has the last electron placed in a d orbital? a)main group elements b)transition elements c)inner transition elements d)nonmetals
Answers
The transition elements have the last electron placed in a d orbital.
In the atoms of the main group elements, the valence electrons are placed in the s and p orbitals. The valence electrons of the nonmetals are located in the p orbitals, while those of the inner transition elements are placed in the f orbitals. The last electron in transition elements is placed in a d orbital.
The electronic configuration of transition elements is characterized by the partially filled d-orbitals. Transition elements comprise the metals, which occupy the central portion of the periodic table and have a valence electron configuration that includes a partially filled d-subshell.
The electrons that are involved in the bond formation are valence electrons, and the d-orbitals are not a part of the valence shell. So, the transition elements exhibit variable oxidation states, and they are good conductors of heat and electricity.
n conclusion, the option that has the last electron placed in a d orbital is transition elements, as it has the electron configuration of (n-1)d1-10ns1-2.
Learn more about valence shell from the given link:
https://brainly.com/question/21729239
#SPJ11
The number of molecules in 48.0 L of oxygen gas (O₂) is --
Answers
There are approximately 1.290 x 10^24 molecules in 48.0 L of oxygen gas (O₂).
To determine the number of molecules in a given volume of gas, we need to use the ideal gas law and Avogadro's principle. The ideal gas law states that PV = nRT, where P is the pressure, V is the volume, n is the number of moles, R is the gas constant, and T is the temperature in Kelvin. First, let's convert the given volume of 48.0 L to moles. We can assume the temperature and pressure are constant. The molar volume of any gas at standard temperature and pressure (STP) is 22.4 L/mol.
48.0 L / 22.4 L/mol ≈ 2.143 moles
Now, we need to convert moles to molecules. One mole of any substance contains Avogadro's number of molecules, which is approximately 6.022 x 10^23 molecules/mol.
2.143 moles x 6.022 x 10^23 molecules/mol ≈ 1.290 x 10^24 molecules
It's important to note that this calculation assumes ideal gas behavior, which may not be completely accurate under all conditions. Additionally, the number of molecules may vary depending on factors such as temperature and pressure. However, for practical purposes and standard conditions, this calculation provides a reasonable estimate of the number of molecules in the given volume of oxygen gas.
for more questions on molecules
https://brainly.com/question/24191825
#SPJ11
a solution contains 25.0 g ethanol (c2h5oh; molar mass 46.07 g/mol) in 500. g h2o (molar mass 18.02 g/mol) at 23oc. if the vapor pressure of pure h2o at this temperature is 20.57 torr, what is the vapor pressure of the solution? multiple choice question. 20.1 torr 21.0 torr 0.390 torr 0.979 torr
Answers
As per Raoult's law the vapor pressure of the solution is given by the term as 0.39 torr, option C.
François-Marie Raoult, a French chemist, discovered during an experiment that when chemicals were combined in a solution, the solution's vapour pressure reduced concurrently. Raoult's law was named in his honour.
Adding a solute decreases vapour pressure because the extra solute particles will fill the spaces left by the solvent particles and occupy space, according to a study of the ideas of collab-rative characteristics. This results in a reduced vapour pressure because there will be less solvent on the surface and less solvent that may escape to enter the gas phase. There are two methods to illustrate how Raoult's Law operates: a straightforward visual method and a more complex one based on entropy. Here is the straightforward method.
According to Raoult's law, a solution's partial pressure equals the sum of its mole fraction of solute and its partial pressure of pure solvent.
Hence;
Partial pressure of the pure solvent = 20.57 torr
Moles of water,
= 500 g/18 g/mol
= 27.8 moles
Moles of ethanol,
= 25.0 g/46 g/mol
= 0.54 moles
Total number of moles = 27.8 moles + 0.54 moles = 28.34 moles
Partial pressure of solution
= 0.54 moles/28.34 moles x 20.57 torr
= 0.39 torr
Therefore, the vapor pressure is 0.39 torr.
Learn more about Raoult's law:
https://brainly.com/question/28304759
#SPJ4
which set of quantum numbers cannot specify an orbital? which set of quantum numbers cannot specify an orbital? n
Answers
n=2,l=1,ml=1 n=3,l=2,ml=0 n=3,l=3,ml=2 n=4,l=2,ml=0.set of quantum numbers cannot specify an orbital.
a zone around a nucleus in an atom or molecule that can have 0–1–2 electrons, as stated theoretically. Around the nucleus, in structures known as orbitals, electrons arrange themselves into clouds. These orbitals are given numbers to represent their energy level and letters (s, p, d, and f) to represent their shape. S, P, D, and f are the four fundamental types of orbitals. Two electrons can fit into a s orbital, which is spherical in shape. There are three p orbitals, each of which is oriented differently in space but has the same fundamental geometry of a . Six electrons can fit in the p orbitals at once.
Learn more about orbital here:
https://brainly.com/question/18914648
#SPJ4
Give an example of how knowledge of physical properties of matter can be used in everyday life
Answers
Understanding physical properties of matter is essential in everyday life for a variety of purposes, from cooking to choosing materials.
Knowledge of physical properties of matter is extremely important in everyday life as it helps us understand the nature of substances we come into contact with. One example is the use of boiling points in cooking. Different substances have different boiling points which determine the temperature at which they boil. This information is crucial in determining cooking times and ensuring that food is cooked properly.
For instance, water boils at 100 degrees Celsius, while sugar syrup boils at a much higher temperature. If the wrong temperature is used, food may be undercooked or overcooked, leading to undesired outcomes. Knowledge of physical properties also helps in choosing the right materials for different purposes, such as choosing heat-resistant materials for cooking.
In conclusion, understanding physical properties of matter is essential in everyday life for a variety of purposes, from cooking to choosing materials.
To know more about matter visit:
brainly.com/question/28487167
#SPJ11
What caused the shielding effect to remain constant across a period
Answers
Answer:
When moving from left to the right of a period, the number of electrons increases and the strength of shielding increases. As you move across period the number of shells remain same, the shielding effect will also remain constant.
Explanation:
Consider this reaction. What volume of oxygen gas, in milliliters, is required to react with 0.640 g of SO2 gas at S TP? 11.2 mL 22.4 mL 112 mL 224 mL

Answers
Answer:
112mL
Explanation:
We'll begin by calculating the number of mole in 0.640g of SO2.
This is illustrated below:
Molar mass of SO2 = 32 + (16x2) = 64g/mol
Mass of SO2 = 0.640g
Number of mole of SO2 =.?
Mole = mass /molar mass
Number of mole of SO2 = 0.640/64
Number of mole of SO2 = 0.01 mole
Next, we shall determine the number of mole of O2 required for the reaction. This is illustrated below:
2SO2(g) + O2(g) —> 2SO3(g)
From the balanced equation above,
2 moles of SO2 reacted with 1 mole of O2.
Therefore, 0.01 mole of SO2 will react with = (0.01 x 1)/2 = 0.005 mole of O2.
Therefore, 0.005 mole of O2 is required for the reaction.
Finally, we shall determine the volume of O2 required for the reaction as follow:
Note: 1 mole of a gas occupy 22.4L (22400mL) at stp.
1 mole of O2 occupy 22400mL at stp.
Therefore, 0.005 mole of O2 will occupy = 0.005 x 22400 = 112mL
Therefore, 112mL of O2 is required for the reaction.
Answer:
the answer is C
Explanation:
i did the test on edg and got it right
Which of these is not a mixture?
A) Salt
B) Cooking oil
C) Tea leaves
D) Milk
ty
Answers
Answer:
the answer is salt because it has a uniform and definite composition
Explanation:
Choose the correct answer:
In a galvanic cell:
a- The reactions taking place are non spontaneous
b- The cathode is negative
c- Electrical energy is converted into chemical energy
d- Chemical energy is converted into electrical energy
Answers
Answer:
D.
Explanation:
In a Galvanic cell:
1) Spontaneous reactions take place
2) Chemical Energy is converted to some useful electrical energy
which group of element which reacts slowly with water to form alkaline solution